Label: GELUSIL- alumina, magnesia, simethicone tablet, chewable
- NDC Code(s): 65197-300-10, 65197-300-11
- Packager: WellSpring Pharmaceutical Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
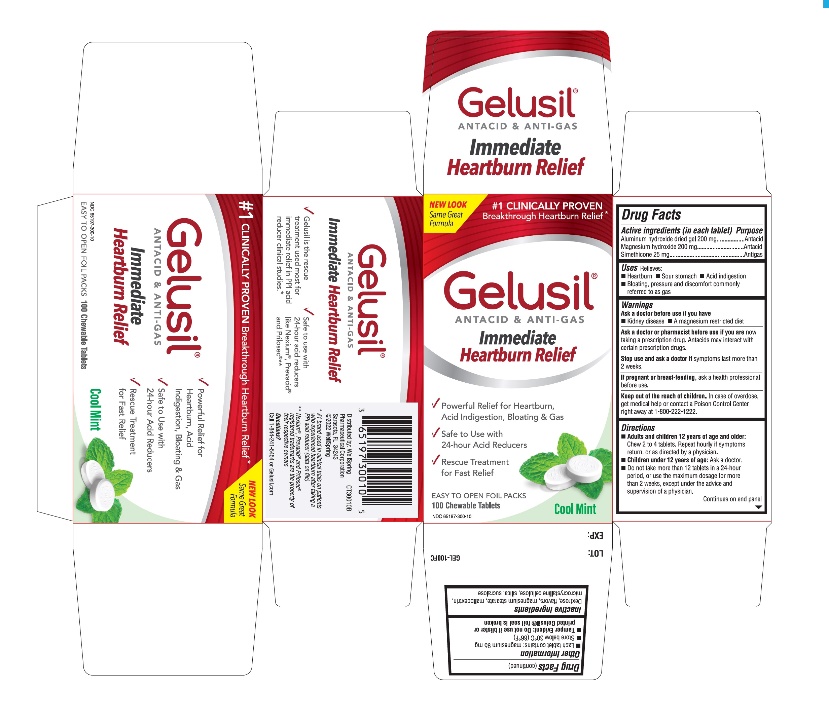
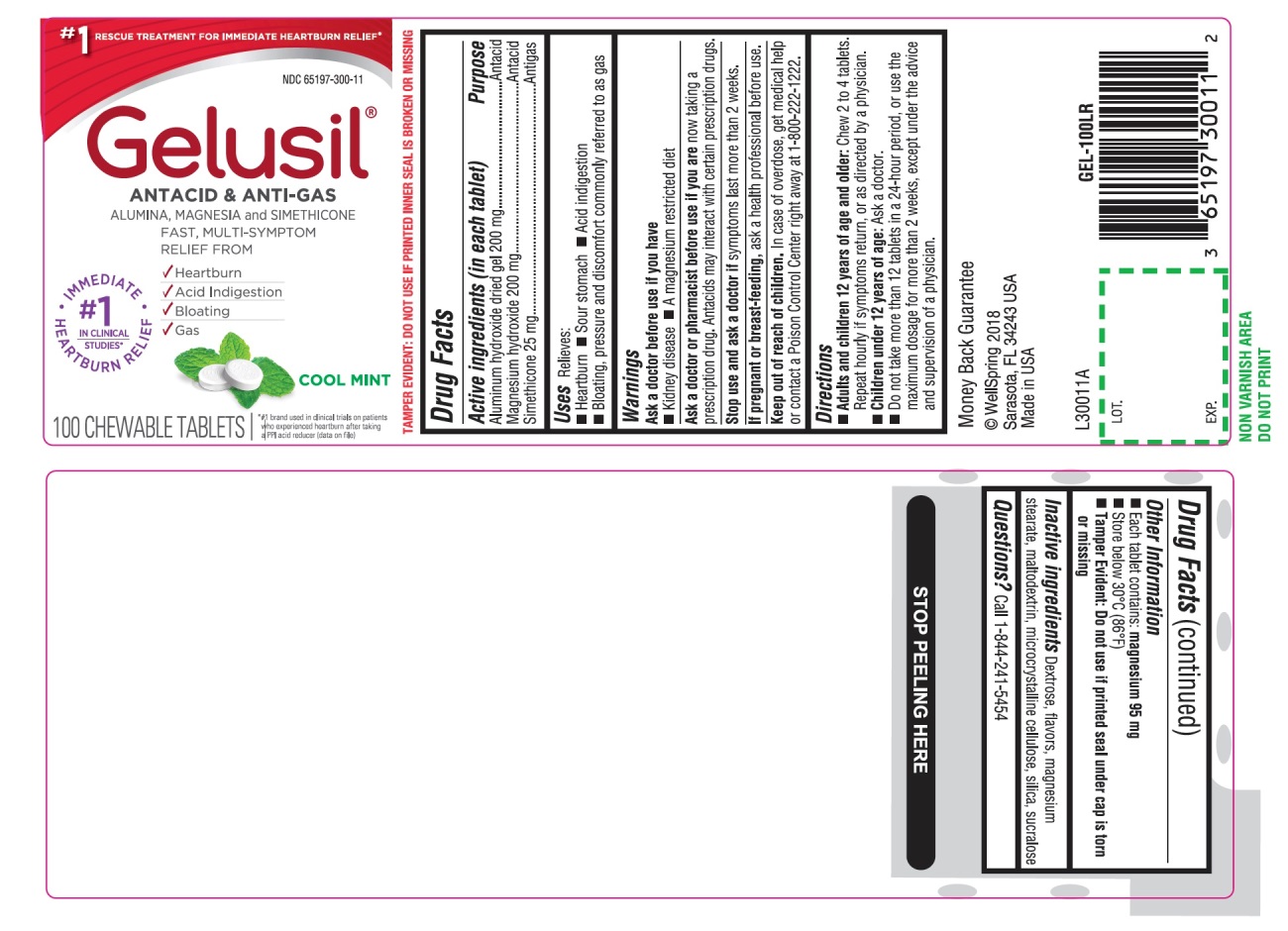
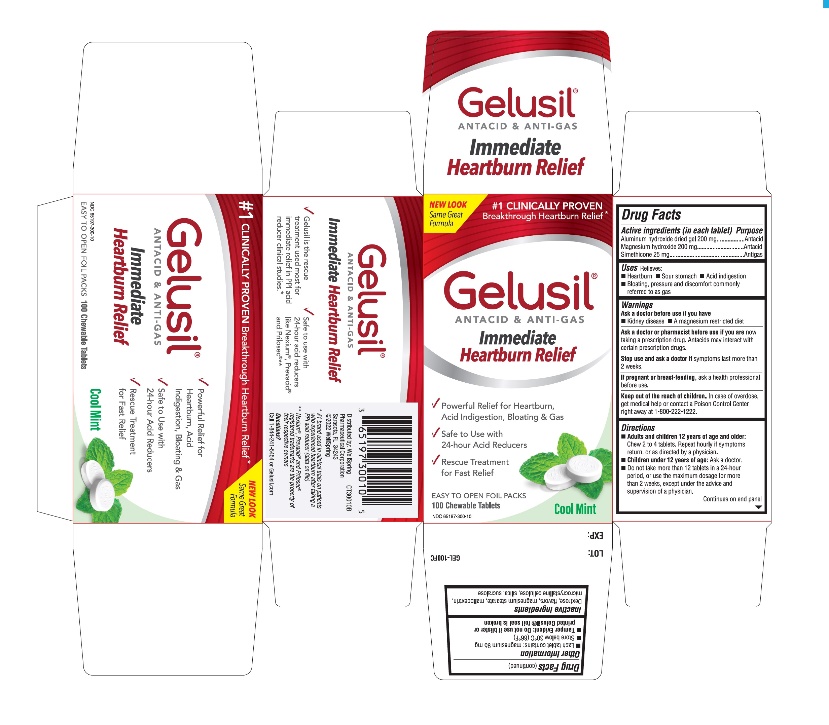
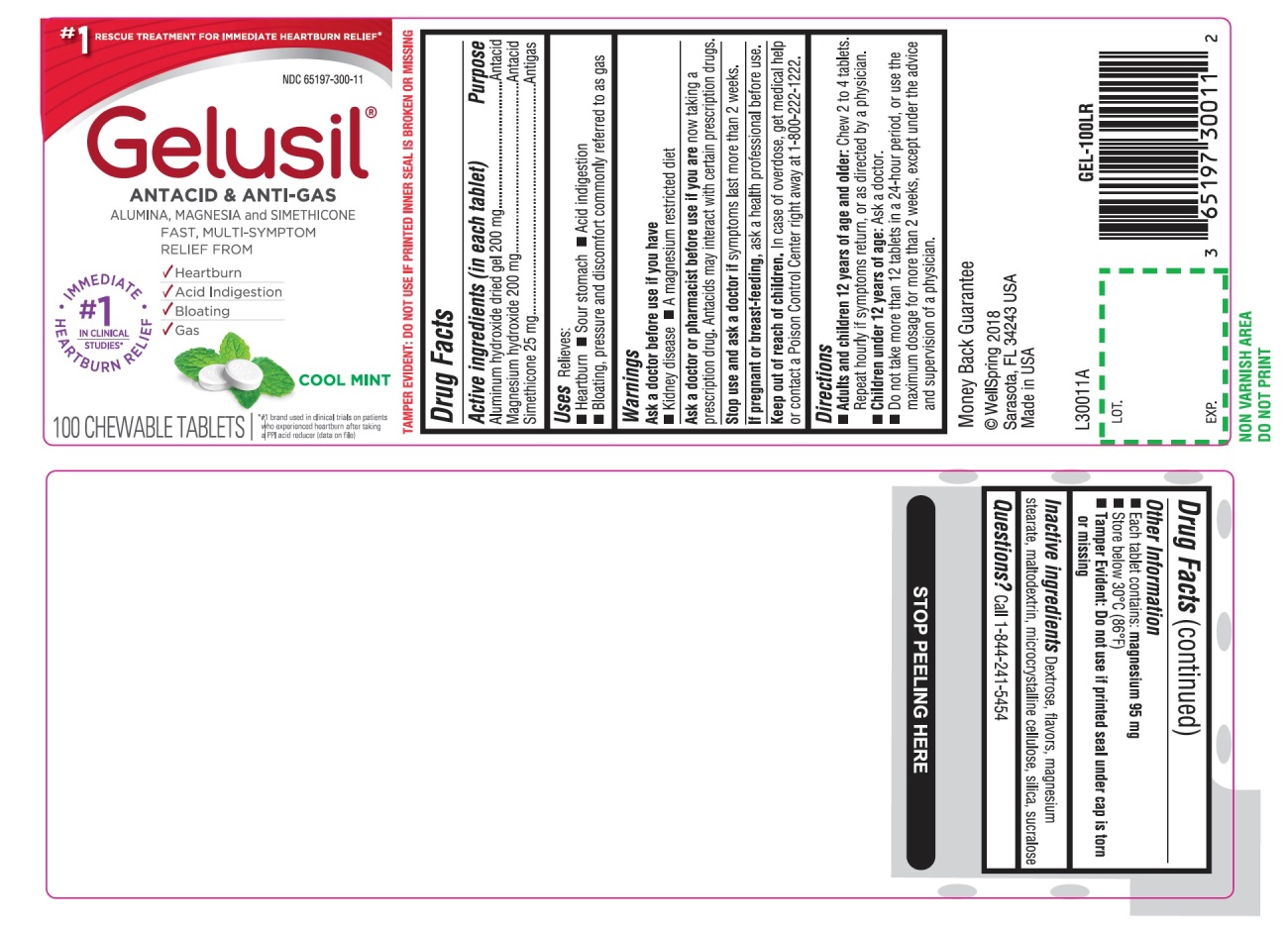
- Active Ingredients (in each tablet)
- Purpose
- Uses
- Warnings
-
Directions
- •
- Adults and children 12 years of age and older: Chew 2 to 4 tablets. Repeat hourly if symptoms return, or as directed by a physician
- •
- Children under 12 of age: ask a doctor
- •
- Do not take more than 12 tablets in a 24-hour period, or use the maximum dosage for more than 2 weeks, except under the advice and supervision of a physician
- Other information
- Inactive ingredients
- Questions?
- Principal display panel - Carton
-
INGREDIENTS AND APPEARANCE
GELUSIL
alumina, magnesia, simethicone tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65197-300 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) (ALUMINUM HYDROXIDE - UNII:5QB0T2IUN0) ALUMINUM HYDROXIDE 200 mg MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM HYDROXIDE 200 mg DIMETHICONE, UNSPECIFIED (UNII: 92RU3N3Y1O) (DIMETHICONE, UNSPECIFIED - UNII:92RU3N3Y1O) DIMETHICONE, UNSPECIFIED 24 mg SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 1 mg Inactive Ingredients Ingredient Name Strength DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) SUCRALOSE (UNII: 96K6UQ3ZD4) SILICON (UNII: Z4152N8IUI) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color WHITE Score no score Shape ROUND Size 16mm Flavor PEPPERMINT Imprint Code PD;GELUSIL;034 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65197-300-10 10 in 1 CARTON 09/24/2008 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:65197-300-11 100 in 1 BOTTLE; Type 0: Not a Combination Product 12/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 09/24/2008 Labeler - WellSpring Pharmaceutical Corporation (110999054)