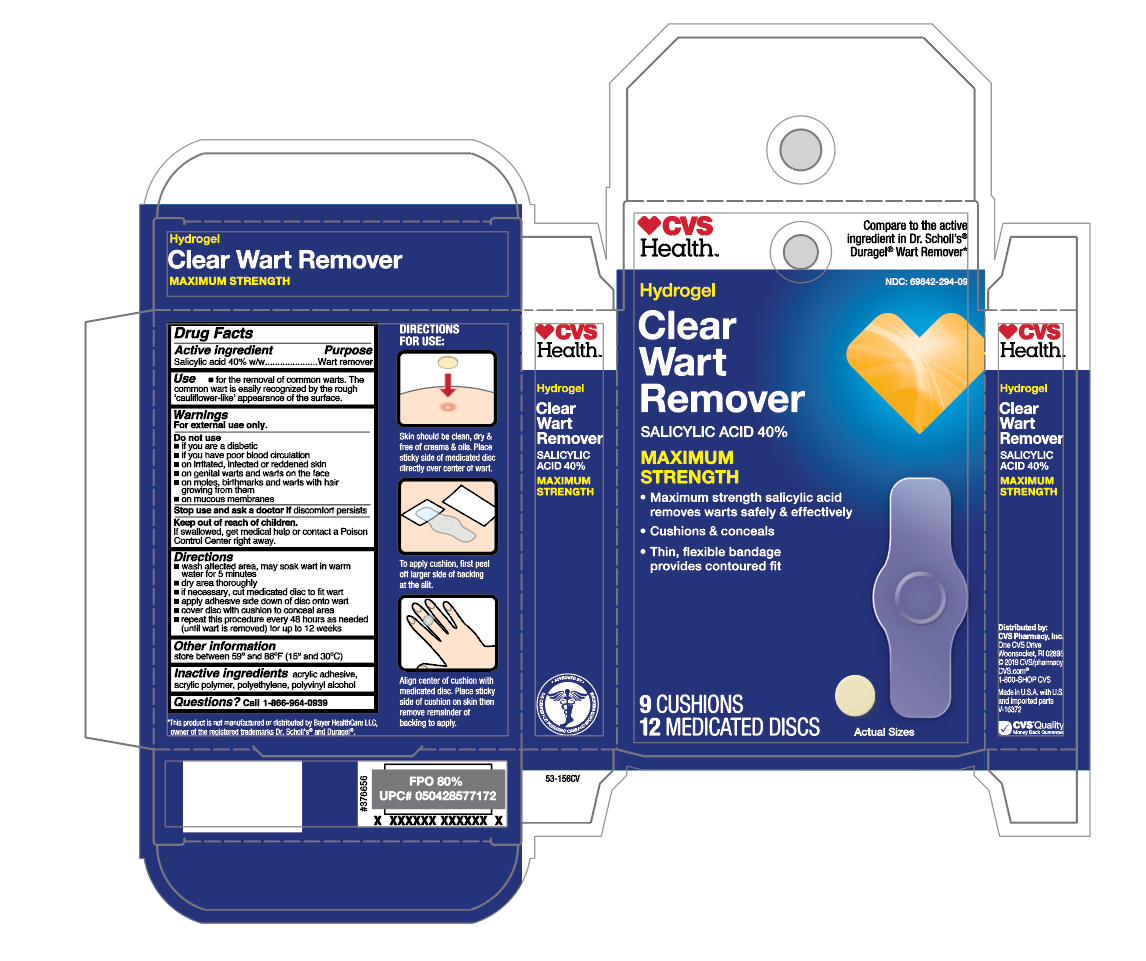
Label: SALICYLIC ACID- hydrogel clear wart remover patch
- NDC Code(s): 69842-294-09
- Packager: CVS Pharmacy
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Use
- Warnings
-
Directions
- wash affected area, may soak wart in warm water for 5 minutes
- dry area thoroughly
- if necessary, cut medicated disc to fit wart
- apply adhesive side down of disc onto wart
- cover disc with cushions to conceal area
- repeat this procedure every 48 hours as needed (until wart is removed) for up to 12 weeks
- Other information
- Inactive ingredients
- Questions?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SALICYLIC ACID
hydrogel clear wart remover patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-294 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 40 mg in 12 Inactive Ingredients Ingredient Name Strength HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) VINYL ACETATE (UNII: L9MK238N77) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-294-09 12 in 1 PACKAGE; Type 0: Not a Combination Product 02/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M028 02/01/2020 Labeler - CVS Pharmacy (062312574)