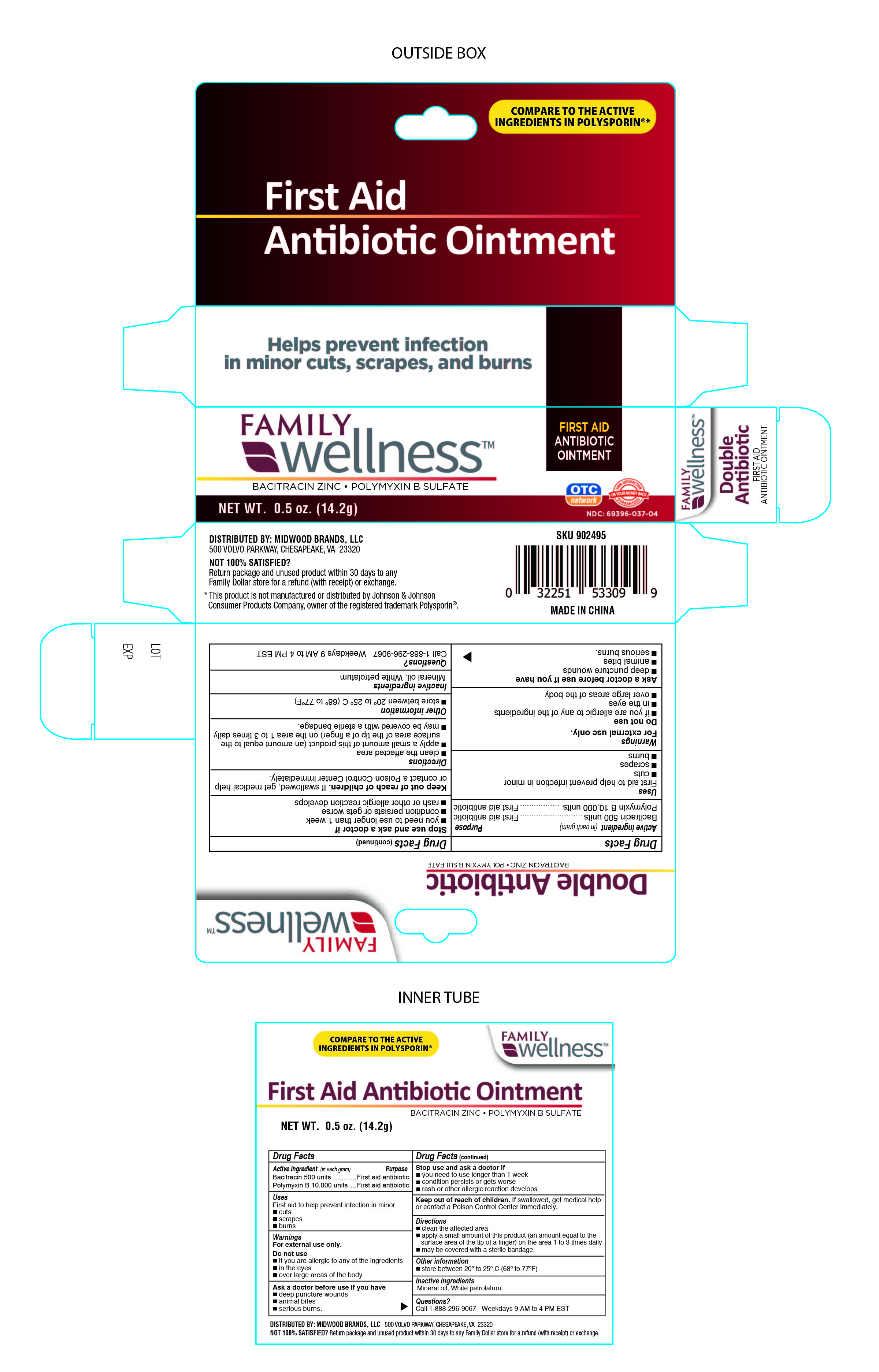
Label: FAMILY WELLNESS FIRST AID ANTIBIOTIC- family wellness bacitracin zinc, polymyxin b sulfate ointment
- NDC Code(s): 69396-037-04, 69396-037-05
- Packager: Trifecta Pharmaceuticals USA, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Uses
- Warnings
- Stop use and ask doctor if:
- KEEP OUT OF REACH OF CHILDREN
- Directions:
- Other Information
- Inactive Ingredients
-
Questions or Comments?
Call 1-888-296-9067 Weekdays 9AM to 4PM EST
DISTRIBUTED BY: MIDWOOD BRANDS, LLC.
500 Volvo Parkway, Chesapeake, VA. 23320
NOT SATISFIED?
Return package and unused product within 30 days to any Family Dollar store for a refund (with receipt) or exchange.
This Product is not manufactured or distributed by Johnson & Johnson Consumer Products Company, owner of the registered trademark Polysporin®
MADE IN CHINA
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FAMILY WELLNESS FIRST AID ANTIBIOTIC
family wellness bacitracin zinc, polymyxin b sulfate ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69396-037 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 10000 [USP'U] in 1 g BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 500 [USP'U] in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) MINERAL OIL (UNII: T5L8T28FGP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69396-037-05 1 in 1 BOX 08/30/2019 1 15 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:69396-037-04 1 in 1 BOX 01/10/2020 2 14.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M004 08/29/2019 Labeler - Trifecta Pharmaceuticals USA, LLC (079424163)