Label: CLOBETASOL PROPIONATE cream
- NDC Code(s): 51672-1297-1, 51672-1297-2, 51672-1297-3
- Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 23, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CLOBETASOL PROPIONATE CREAM (EMOLLIENT) safely and effectively. See full prescribing information for CLOBETASOL PROPIONATE CREAM ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEClobetasol propionate cream, 0.05% (emollient) is a super-high potency corticosteroid indicated for: 1.1 Corticosteriod-Responsive Dermatoses - Clobetasol propionate cream, 0.05% (emollient) is ...
-
2 DOSAGE AND ADMINISTRATIONApply a thin layer of clobetasol propionate cream, 0.05% (emollient) to the affected skin areas twice daily and rub in gently and completely. Wash hands after each application. Clobetasol ...
-
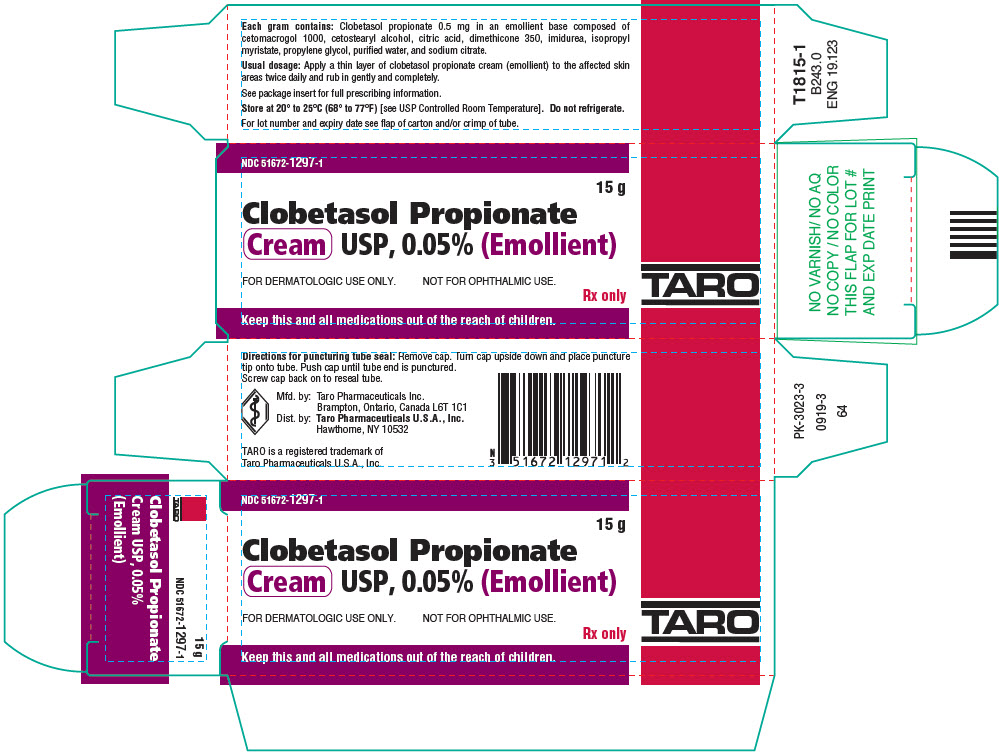
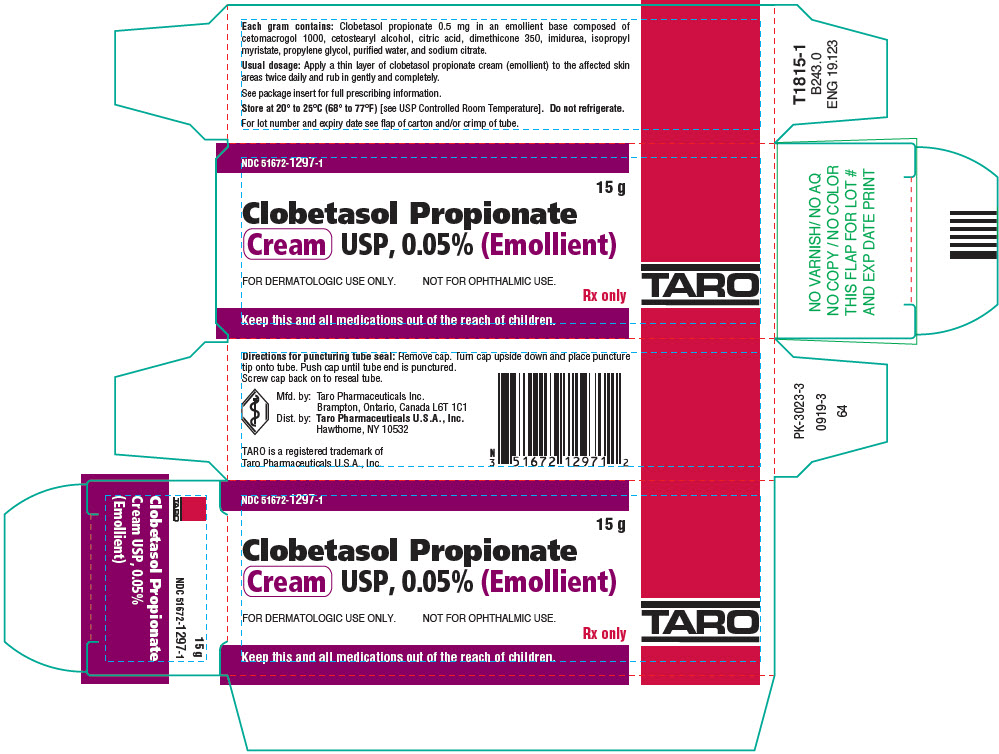
3 DOSAGE FORMS AND STRENGTHSCream, 0.05%. Each gram of Clobetasol Propionate Cream USP, 0.05% (Emollient) contains 0.5 mg of clobetasol propionate in a white to off-white cream base.
-
4 CONTRAINDICATIONSNone
-
5 WARNINGS AND PRECAUTIONS5.1 Effects on the Endocrine System - Clobetasol propionate is a highly potent topical corticosteroid that has been shown to suppress the HPA axis at doses as low as 2 grams per day. Systemic ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - There are no adequate and well-controlled studies in pregnant women. Therefore, clobetasol propionate cream, 0.05% (emollient) should be used during pregnancy only if the ...
-
10 OVERDOSAGETopically applied clobetasol propionate cream, 0.05% (emollient) can be absorbed in sufficient amounts to produce systemic effects [see - Warnings and Precautions (5.1)].
-
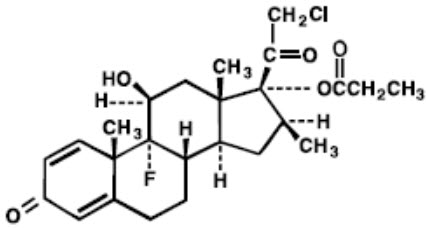
11 DESCRIPTIONClobetasol Propionate Cream USP, 0.05% (Emollient) contains the active compound clobetasol propionate, a synthetic corticosteroid, for topical use. Clobetasol, an analog of prednisolone, has a ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Like other topical corticosteroids, clobetasol propionate has anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory ...
-
13 NONCLINCAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long-term animal studies have not been performed to evaluate the carcinogenic potential of clobetasol ...
-
14 CLINICAL STUDIESIn a controlled clinical trial involving patients with moderate to severe plaque-type psoriasis, clobetasol propionate cream, 0.05% (emollient) was applied to 5% to 10% of body surface area. In ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - Clobetasol Propionate Cream USP, 0.05% (Emollient) is a white to off-white cream, supplied in 15 g (NDC 51672-1297-1), 30 g (NDC 51672-1297-2) and 60 g tubes (NDC ...
-
17 PATIENT COUNSELING INFORMATIONInform patients using topical corticosteroids of the following information and instructions: Clobetasol propionate cream, 0.05% (emollient) is for external use only. Avoid contact with the ...
-
SPL UNCLASSIFIED SECTIONMfd. by: Taro Pharmaceuticals Inc. Brampton, Ontario, Canada L6T 1C1 - Dist. by: Taro Pharmaceuticals U.S.A., Inc., Hawthorne, NY 10532 - Revised: September 2019 - PK-3030-3 ...
-
PRINCIPAL DISPLAY PANEL - 15 g Tube CartonNDC 51672-1297-1 - 15 g - Clobetasol Propionate - Cream USP, 0.05% (Emollient) FOR DERMATOLOGIC USE ONLY. NOT FOR OPHTHALMIC USE. Rx only - Keep this and all medications out of the reach ...
-
INGREDIENTS AND APPEARANCEProduct Information