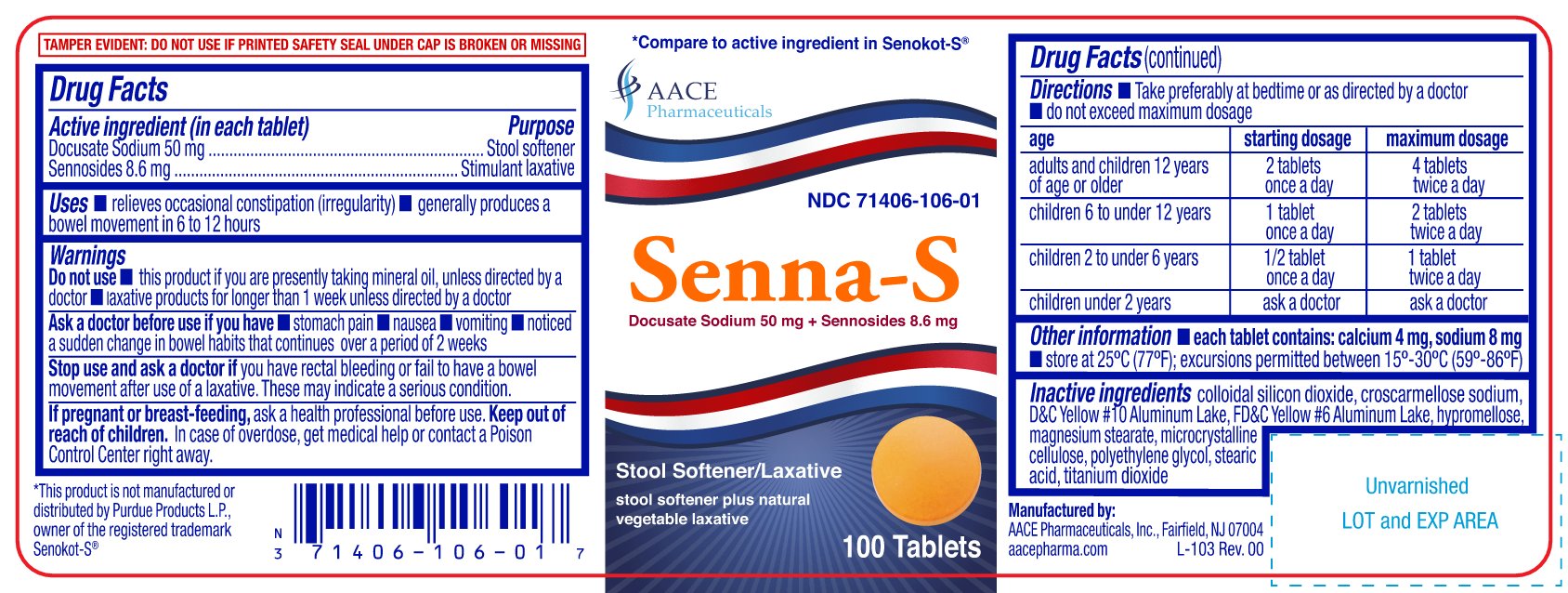
Label: SENNA-S- docusate sodium 50 mg sennosides 8.6 mg tablet, film coated
- NDC Code(s): 71406-106-01, 71406-106-10
- Packager: AACE PHARMACEUTICALS, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT (IN EACH TABLET)
- PURPOSE
- USES
-
WARNINGS
Do not use
- this product if you are presently taking mineral oil, unless directed by a doctor
- laxative products for longer than 1 week unless directed by a doctor
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that continues over a period of 2 weeks
-
DIRECTIONS
- take preferably at bedtime or as directed by a doctor
- do not exceed maximum dosage
age starting dosage maximum dosage adults and children 12 years of age or older 2 tablets once a day 4 tablets twice a day children 6 to under 12 years 1 tablet once a day 2 tablets twice a day children 2 to under 6 years 1/2 tablet once a day 1 tablet twice a day children under 2 years ask a doctor ask a doctor - OTHER INFORMATION
- INACTIVE INGREDIENTS
-
PRINCIPAL DISPLAY PANEL
Senna-S
Docusate Sodium 50 mg & Sennosides 8.6 mg
NDC 71406-106-01, 71406-106-10
Stool Softner/Laxative
stool softner plus natural vegetable laxative
*Compare to the active ingredients in SENOKOT-S ®
TEMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Manufactured by:
AACE Pharmaceuticals, Inc. Fairfield, NJ 07004
aacepharma.com
*This product is not manufactured or distributed by Purdue Products L.P., owner of the registered trademark Senokot-S ®.

-
INGREDIENTS AND APPEARANCE
SENNA-S
docusate sodium 50 mg sennosides 8.6 mg tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71406-106 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 50 mg SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.6 mg Inactive Ingredients Ingredient Name Strength SODIUM BENZOATE (UNII: OJ245FE5EU) MAGNESIUM STEARATE (UNII: 70097M6I30) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 4000 (UNII: 4R4HFI6D95) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) Product Characteristics Color orange Score no score Shape ROUND Size 9mm Flavor Imprint Code S6 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71406-106-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/30/2019 2 NDC:71406-106-10 1000 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/30/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 08/30/2019 Labeler - AACE PHARMACEUTICALS, INC. (080630748)