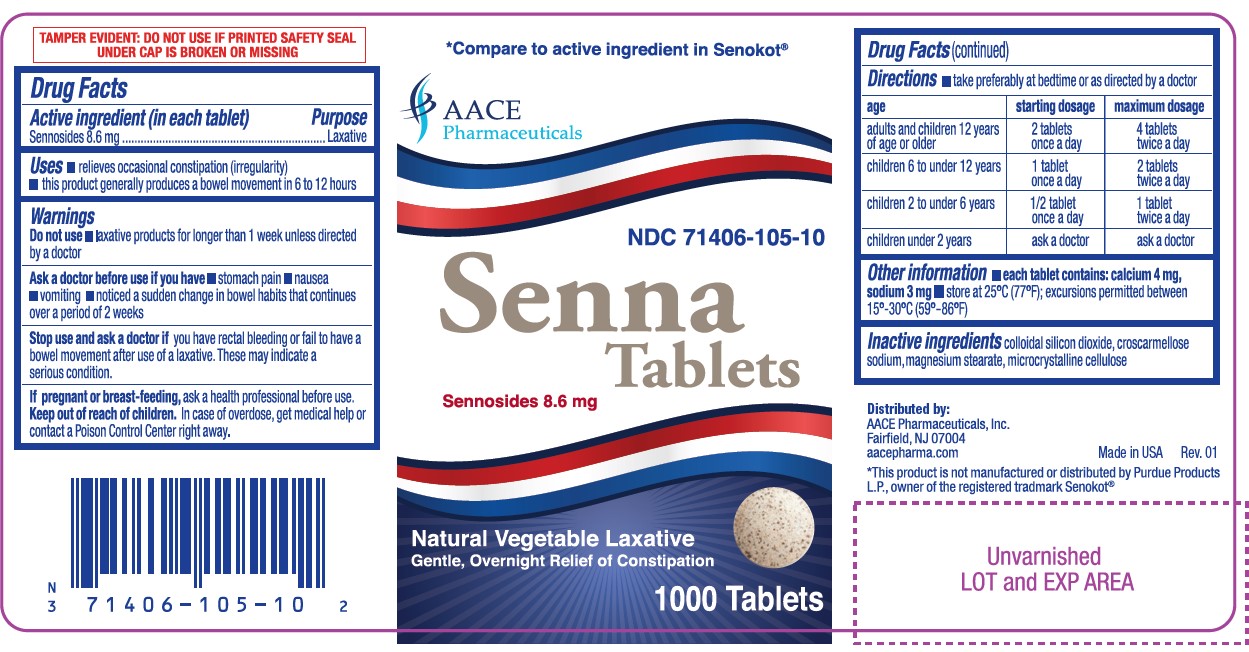
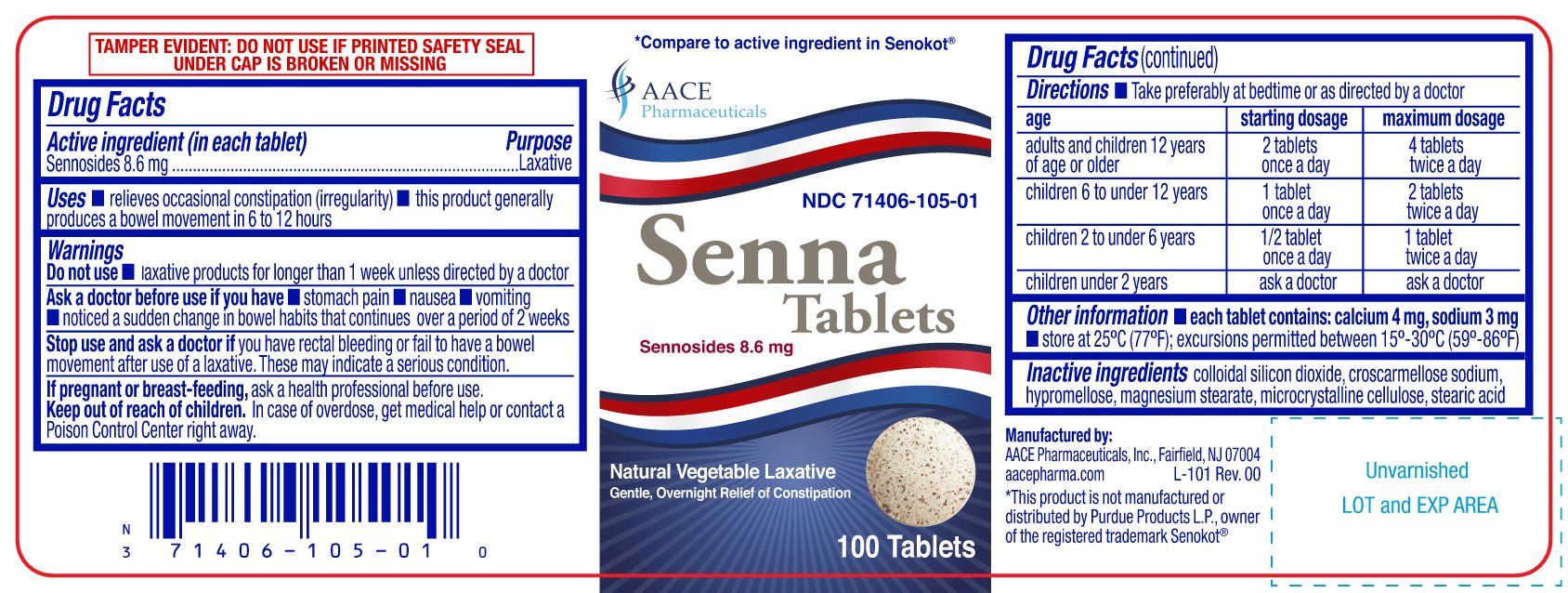
Label: SENNA- sennosides 8.6 mg tablets tablet
- NDC Code(s): 71406-105-01, 71406-105-10
- Packager: AACE PHARMACEUTICALS, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT (IN EACH TABLET)
- PURPOSE
- USES
- WARNINGS
-
DIRECTIONS
- take preferably at bedtime or as directed by a doctor
age starting dosage maximum dosage adults and children 12 years of age or older 2 tablets once a day 4 tablets twice a day children 6 to under 12 years 1 tablet once a day 2 tablets twice a day children 2 to under 6 years 1/2 tablet once a day 1 tablet twice a day children under 2 years ask a doctor ask a docto - OTHER INFORMATION
- INACTIVE INGREDIENTS
-
PRINCIPAL DISPLAY PANEL
†Compare to the active ingredient in Senokot®
Senna Tablets
Sennosides 8.6 mg
Natural Vegetable Laxative
Gentle, Overnight Relief of Constipation
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Manufactured by:
AACE Pharmaceuticals, Inc. Fairfield, NJ 07004
aacepharma.com
†This product is not manufactured or distributed by Purdue Products L.P., owner of the registered trademark Senokot®.
-
INGREDIENTS AND APPEARANCE
SENNA
sennosides 8.6 mg tablets tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71406-105 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.6 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color brown (Light brown to grey) Score score with uneven pieces Shape ROUND Size 9mm Flavor Imprint Code S5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71406-105-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/30/2019 2 NDC:71406-105-10 1000 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/30/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 08/30/2019 Labeler - AACE PHARMACEUTICALS, INC. (080630748)


