Label: BENAZEPRIL HYDROCHLORIDE tablet
- NDC Code(s): 23155-749-01, 23155-749-05, 23155-750-01, 23155-750-05, view more
- Packager: Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 27, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BENAZEPRIL HYDROCHLORIDE TABLETS safely and effectively. See full prescribing information for use BENAZEPRIL HYDROCHLORIDE ...
-
Table of ContentsTable of Contents
- BOXED WARNING (What is this?)
-
1 INDICATIONS AND USAGEBenazepril hydrochloride is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - ADULTS - The recommended initial dose for patients not receiving a diuretic is 10 mg once a day. The usual maintenance dosage range is 20 to 40 mg per day administered ...
-
3 DOSAGE FORMS AND STRENGTHSTablets: 5 mg, 10 mg, 20 mg, and 40 mg - Each 5 mg tablet is light orange, round, film-coated tablets debossed "696" on one side and plain on the other side. Each 10 mg tablet is ...
-
4 CONTRAINDICATIONSBenazepril hydrochloride is contraindicated in patients: who are hypersensitive to benazepril or to any other ACE inhibitor - with a history of angioedema with or without previous ACE inhibitor ...
-
5 WARNINGS AND PRECAUTIONS5.1 Fetal Toxicity - Benazepril hydrochloride can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third ...
-
6 ADVERSE REACTIONSBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical ...
-
7 DRUG INTERACTIONS7.1 Diuretics - Hypotension - Patients on diuretics, especially those in whom diuretic therapy was recently instituted, may occasionally experience an excessive reduction of blood pressure after ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Benazepril hydrochloride can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and ...
-
10 OVERDOSAGESingle oral doses of 3 g/kg benazepril were associated with significant lethality in mice. Rats, however, tolerated single oral doses of up to 6 g/kg. Reduced activity was seen at 1 g/kg in mice ...
-
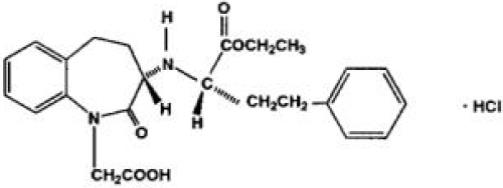
11 DESCRIPTIONBenazepril hydrochloride, USP is a white to off-white crystalline powder, soluble (>100 mg/mL) in water, in ethanol, and in methanol. Its chemical name is benazepril 3-[[1-(ethoxy-carbonyl)-3 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Benazepril and benazeprilat inhibit angiotensin-converting enzyme (ACE) in human subjects and animals. Benazeprilat has much greater ACE inhibitory activity than does ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No evidence of carcinogenicity was found when benazepril was administered to rats and mice for up to two years at doses of up to 150 ...
-
14 CLINICAL STUDIESHypertension - Adult Patients - In single-dose studies, benazepril hydrochloride lowered blood pressure within 1 hour, with peak reductions achieved between 2 and 4 hours after dosing. The ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGBenazepril Hydrochloride Tablets USP, 5 mg are available as light orange, round, film-coated tablets debossed "696" on one side and plain on the other side containing 5 mg benazepril ...
-
17 PATIENT COUNSELING INFORMATIONPregnancy: Tell female patients of childbearing age about the consequences of exposure to benazepril hydrochloride during pregnancy. Discuss treatment options with women planning to become ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 23155-749-01 - Benazepril Hydrochloride Tablets, USP - Rx Only - 5 mg, 100 tablets. NDC 23155-750-01 - Benazepril Hydrochloride Tablets, USP - Rx Only - 10 mg ...
-
INGREDIENTS AND APPEARANCEProduct Information