Label: METFORMIN HYDROCHLORIDE tablet
- NDC Code(s): 70518-3735-0
- Packager: REMEDYREPACK INC.
- This is a repackaged label.
- Source NDC Code(s): 50228-105
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 28, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use METFORMIN HYDROCHLORIDE TABLETS safely and effectively. See full prescribing information for METFORMIN HYDROCHLORIDE TABLETS.
METFORMIN HYDROCHLORIDE tablets, for oral use
Initial U.S. Approval: 1995DOSAGE FORMS AND STRENGTHS
Metformin Hydrochloride Tablets, USP: 500 mg, 850 mg, and 1000 mg ( 3) (1)
ADVERSE REACTIONS
For Metformin Hydrochloride Tablets, the most common adverse reactions (>5.0%) are diarrhea, nausea/vomiting, flatulence, asthenia, indigestion, abdominal discomfort, and headache. ( 6.1) (2)
To report SUSPECTED ADVERSE REACTIONS, contact ScieGen at (855) 724-3436 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch (2)
DOSAGE AND ADMINISTRATION
2 DOSAGE AND ADMINISTRATION (12)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
3 DOSAGE FORMS AND STRENGTHS
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Metformin Hydrochloride Tablets
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
3 DOSAGE FORMS AND STRENGTHS
Metformin Hydrochloride Tablets, USP are available as:
Metformin Hydrochloride Tablets, USP 500 mg are blackberry flavored, white to off-white, round, biconvex, beveled edge film coated tablets, debossed with ‘SG’ on one side ‘105’ on other side.
Metformin Hydrochloride Tablets, USP 850 mg are blackberry flavored, white to off-white, round, biconvex, beveled edge film coated tablets, debossed with ‘SG’ on one side ‘106’ on other side.
Metformin Hydrochloride Tablets, USP 1000 mg tablets are blackberry flavored, white to off-white, oval, biconvex, film coated tablets debossed on one side with S on the left side of bisect and G on the right side of bisect and other side 1 on the left side and 07 on the right side of the bisect. -
6 ADVERSE REACTIONS
The following adverse reactions are also discussed elsewhere in the labeling:
Lactic Acidosis [ see Boxed Warning and Warnings and Precautions (5.1)]
Vitamin B 12 Deficiency [ see Warnings and Precautions (5.2)]
Hypoglycemia [ see Warnings and Precautions (5.3)]6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Metformin Hydrochloride Tablets
In a U.S. clinical trial of Metformin Hydrochloride in patients with type 2 diabetes mellitus, a total of 141 patients received Metformin Hydrochloride up to 2550 mg per day. Adverse reactions reported in greater than 5% of Metformin Hydrochloride treated patients and that were more common than in placebo-treated patients, are listed in Table 1.
Table 1: Adverse Reactions from a Clinical Trial of Metformin Hydrochloride Occurring >5% and More Common than Placebo in Patients with Type 2 Diabetes Mellitus
Metformin
Hydrochloride
(n=141)
Placebo
(n=145)
Diarrhea
53%
12%
Nausea/Vomiting
26%
8%
Flatulence
12%
6%
Asthenia
9%
6%
Indigestion
7%
4%
Abdominal Discomfort
6%
5%
Headache
6%
5%
Diarrhea led to discontinuation of Metformin Hydrochloride in 6% of patients. Additionally, the following adverse reactions were reported in ≥1% to ≤5% of Metformin Hydrochloride treated patients and were more commonly reported with Metformin Hydrochloride than placebo: abnormal stools, hypoglycemia, myalgia, lightheaded, dyspnea, nail disorder, rash, sweating increased, taste disorder, chest discomfort, chills, flu syndrome, flushing, palpitation.
In Metformin Hydrochloride clinical trials of 29-week duration, a decrease to subnormal levels of previously normal serum vitamin B 12 levels was observed in approximately 7% of patients.
Pediatric Patients
In clinical trials with Metformin Hydrochloride Tablets in pediatric patients with type 2 diabetes mellitus, the profile of adverse reactions was similar to that observed in adults.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of metformin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cholestatic, hepatocellular, and mixed hepatocellular liver injury have been reported with postmarketing use of metformin.
-
10 OVERDOSAGE
Overdose of metformin hydrochloride has occurred, including ingestion of amounts greater than 50 grams. Hypoglycemia was reported in approximately 10% of cases, but no causal association with metformin has been established. Lactic acidosis has been reported in approximately 32% of metformin overdose cases [ see Warnings and Precautions (5.1) ]. Metformin is dialyzable with a clearance of up to 170 mL/min under good hemodynamic conditions. Therefore, hemodialysis may be useful for removal of accumulated drug from patients in whom metformin overdosage is suspected.
-
11 DESCRIPTION
Metformin Hydrochloride Tablets, USP contain the antihyperglycemic agent metformin, which is a biguanide, in the form of monohydrochloride. The chemical name of metformin hydrochloride is N,N-dimethylimidodicarbonimidic diamide hydrochloride. The structural formula is as shown below
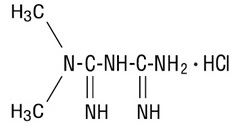
Metformin hydrochloride is a white crystalline compound with a molecular formula of C 4H 11N 5 • HCl and a molecular weight of 165.63. Metformin hydrochloride is freely soluble in water, slightly soluble in alcohol, practically insoluble in acetone and in methylene hydrochloride. The pK a of metformin is 12.4. The p H of a 1% aqueous solution of metformin hydrochloride is 6.68.
Metformin Hydrochloride Tablets contain 500 mg, 850 mg, or 1000 mg of metformin hydrochloride, which is equivalent to 389.93 mg, 662.88 mg, 779.86 mg metformin base, respectively. Each tablet contains the inactive ingredients pregelatinized starch (maize), povidone, crospovidone, magnesium stearate. In addition, the coating for the tablets contains hypromellose, polyethylene glycol, titanium dioxide and flavoring agent contains dextrose, ethyl alcohol, gum arabic, propylene glycol and silicon dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Metformin is an antihyperglycemic agent which improves glucose tolerance in patients with type 2 diabetes mellitus, lowering both basal and postprandial plasma glucose. Metformin decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization. With metformin therapy, insulin secretion remains unchanged while fasting insulin levels and day-long plasma insulin response may decrease.
12.3 Pharmacokinetics
Absorption
The absolute bioavailability of a metformin hydrochloride 500 mg tablet given under fasting conditions is approximately 50% to 60%. Studies using single oral doses of metformin hydrochloride 500 to 1500 mg and 850 to 2550 mg, indicate that there is a lack of dose proportionality with increasing doses, which is due to decreased absorption rather than an alteration in elimination. At usual clinical doses and dosing schedules of metformin hydrochloride, steady state plasma concentrations of metformin are reached within 24 to 48 hours and are generally <1 μg/mL.
Effect of food: Food decreases the extent of absorption and slightly delays the absorption of metformin, as shown by approximately a 40% lower mean peak plasma concentration (C max), a 25% lower area under the plasma concentration versus time curve (AUC), and a 35-minute prolongation of time to peak plasma concentration (T max) following administration of a single 850 mg tablet of Metformin Hydrochloride with food, compared to the same tablet strength administered fasting.
Distribution
The apparent volume of distribution (V/F) of metformin following single oral doses of Metformin Hydrochloride 850 mg averaged 654 ± 358 L. Metformin is negligibly bound to plasma proteins. Metformin partitions into erythrocytes, most likely as a function of time.
Metabolism
Intravenous single-dose studies in normal subjects demonstrate that metformin is excreted unchanged in the urine and does not undergo hepatic metabolism (no metabolites have been identified in humans) nor biliary excretion.
Elimination
Renal clearance (see Table 4) is approximately 3.5 times greater than creatinine clearance, which indicates that tubular secretion is the major route of metformin elimination. Following oral administration, approximately 90% of the absorbed drug is eliminated via the renal route within the first 24 hours, with a plasma elimination half-life of approximately 6.2 hours. In blood, the elimination half-life is approximately 17.6 hours, suggesting that the erythrocyte mass may be a compartment of distribution.
Specific Populations
Renal Impairment
In patients with decreased renal function the plasma and blood half-life of metformin is prolonged and the renal clearance is decreased (see Table 3) [ See Dosage and Administration (2.3), Contraindications (4), Warnings and Precautions (5.1) and Use in Specific Populations (8.6)] .
Hepatic Impairment
No pharmacokinetic studies of metformin have been conducted in patients with hepatic impairment [ See Warnings and Precautions (5.1) and Use in Specific Populations (8.7)].
Geriatrics
Limited data from controlled pharmacokinetic studies of metformin hydrochloride in healthy elderly subjects suggest that total plasma clearance of metformin is decreased, the half-life is prolonged, and C max is increased, compared to healthy young subjects. It appears that the change in metformin pharmacokinetics with aging is primarily accounted for by a change in renal function (see Table 4). [ See Warnings and Precautions (5.1) and Use in Specific Populations (8.5) ].
Table 4: Select Mean (±S.D.) Metformin Pharmacokinetic Parameters Following Single or Multiple Oral Doses of Metformin Hydrochloride Tablets
a All doses given fasting except the first 18 doses of the multiple dose studies
b Peak plasma concentration
c Time to peak plasma concentration
d Combined results (average means) of five studies: mean age 32 years (range 23-59 years)
e Kinetic study done following dose 19, given fasting
f Elderly subjects, mean age 71 years (range 65-81 years)
g CLcr = creatinine clearance normalized to body surface area of 1.73 m 2
Subject Groups:
Metformin hydrochloride tablets dose a
(number of subjects) C max b
(mcg/mL) T max c
(hrs) Renal Clearance
(mL/min)
Healthy, nondiabetic adults:
500 mg single dose (24)
850 mg single dose (74) d
850 mg three times daily for 19 doses e (9)
1.03 (±0.33)
1.60 (±0.38)
2.01 (±0.42)
2.75 (±0.81)
2.64 (±0.82)
1.79 (±0.94)
600 (±132)
552 (±139)
642 (±173)
Adults with type 2 diabetes mellitus:
850 mg single dose (23)
850 mg three times daily for 19 doses e (9)
1.48 (±0.5)
1.90 (±0.62)
3.32 (±1.08)
2.01 (±1.22)
491 (±138)
550 (±160)
Elderly f , healthy nondiabetic adults:
850 mg single dose (12)
2.45 (±0.70)
2.71 (±1.05)
412 (±98)
Renal-impaired adults: 850 mg single dose
Mild (CLcr g 61 to 90 mL/min) (5)
Moderate (CLcr 31 to 60 mL/min) (4)
Severe (CLcr 10 to 30 mL/min) (6)
1.86 (±0.52)
4.12 (±1.83)
3.93 (±0.92)
3.20 (±0.45)
3.75 (±0.50)
4.01 (±1.10)
384 (±122)
108 (±57)
130 (±90)Pediatrics
After administration of a single oral metformin hydrochloride 500 mg tablet with food, geometric mean metformin C max and AUC differed less than 5% between pediatric type 2 diabetic patients (12-16 years of age) and gender-and weight-matched healthy adults (20-45 years of age), all with normal renal function.
Gender
Metformin pharmacokinetic parameters did not differ significantly between normal subjects and patients with type 2 diabetes mellitus when analyzed according to gender (males=19, females=16).
Race
No studies of metformin pharmacokinetic parameters according to race have been performed.
Drug Interactions
In Vivo Assessment of Drug Interactions
Table 5: Effect of Coadministered Drug on Plasma Metformin Systemic Exposure
Coadministered Drug Dose of Coadministered Drug * Dose of Metformin * Geometric Mean Ratio
(ratio with/without coadministered drug)
No Effect = 1.00
AUC † C max
No dosing adjustments required for the following:
Glyburide 5 mg 850 mg metformin 0.91 ‡ 0.93 ‡
Furosemide 40 mg 850 mg metformin 1.09 ‡ 1.22 ‡
Nifedipine 10 mg 850 mg metformin 1.16 1.21
Propranolol 40 mg 850 mg metformin 0.90 0.94
Ibuprofen 400 mg 850 mg metformin 1.05 ‡ 1.07 ‡
Cationic drugs eliminated by renal tubular secretion may reduce metformin elimination
Cimetidine 400 mg 850 mg metformin 1.40 1.61
Carbonic anhydrase inhibitors may cause metabolic acidosis [ See Warnings and Precautions (5.1) and Drug Interactions (7).]
Topiramate 100 mg § 500 mg § metformin 1.25 § 1.07* All metformin and coadministered drugs were given as single doses
† AUC = AUC(INF)
‡ Ratio of arithmetic means
§ At steady state with topiramate 100 mg every 12 hours and metformin 500 mg every 12 hours;
AUC = AUC 0-12hTable 6: Effect of Metformin on Coadministered Drug Systemic Exposure
Coadministered Drug Dose of
Coadministered
;Drug * Dose of
Metformin * Geometric Mean Ratio
(ratio with/without metformin)
No Effect = 1.00
AUC † C max
No dosing adjustments required for the following:
Glyburide 5 mg 850 mg glyburide 0.78 ‡ 0.63 ‡
Furosemide 40 mg 850 mg furosemide 0.87 ‡ 0.69 ‡
Nifedipine 10 mg 850 mg nifedipine 1.10 § 1.08
Propranolol 40 mg propranolol 1.01 § 1.02
r>
Ibuprofen 400 mg 850 mg ibuprofen 0.97 ¶ 1.01 ¶
Cimetidine 400 mg 850 mg cimetidine 0.95 § 1.01* All metformin and coadministered drugs were given as single doses
† AUC = AUC(INF) unless otherwise noted
‡ Ratio of arithmetic means, p-value of difference <0.05
§ AUC(0-24 hr) reported
¶ Ratio of arithmetic means -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity studies have been performed in rats (dosing duration of 104 weeks) and mice (dosing duration of 91 weeks) at doses up to and including 900 mg/kg/day and 1500 mg/kg/day, respectively. These doses are both approximately 3 times the maximum recommended human daily dose of 2550 mg based on body surface area comparisons. No evidence of carcinogenicity with metformin was found in either male or female mice. Similarly, there was no tumorigenic potential observed with metformin in male rats. There was, however, an increased incidence of benign stromal uterine polyps in female rats treated with 900 mg/kg/day.
There was no evidence of a mutagenic potential of metformin in the following in vitro tests: Ames test ( S. typhimurium), gene mutation test (mouse lymphoma cells), or chromosomal aberrations test (human lymphocytes). Results in the in vivo mouse micronucleus test were also negative.
Fertility of male or female rats was unaffected by metformin when administered at doses as high as 600 mg/kg/day, which is approximately 2 times the maximum recommended human daily dose of 2550 mg based on body surface area comparisons.
-
14 CLINICAL STUDIES
14.1 Metformin Hydrochloride Tablets
Adult Clinical Studies
A double-blind, placebo-controlled, multicenter US clinical trial involving obese patients with type 2 diabetes mellitus whose hyperglycemia was not adequately controlled with dietary management alone (baseline fasting plasma glucose [FPG] of approximately 240 mg/dL) was conducted. Patients were treated with Metformin Hydrochloride Tablets (up to 2550 mg/day) or placebo for 29 weeks. The results are presented in Table 7.
Table 7: Mean Change in Fasting Plasma Glucose and HbA1c at Week 29 Comparing Metformin Hydrochloride Tablets vs Placebo in Patients with Type 2 Diabetes Mellitus
Metformin
Hydrochloride
(n=141)
Placebo
(n=145) p-Value
FPG (mg/dL)
Baseline
Change at FINAL VISIT 241.5
–53.0 237.7
6.3 NS *
0.001
Hemoglobin A1c (%)
Baseline
Change at FINAL VISIT 8.4
–1.4 8.2
0.4 NS *
0.001* Not statistically significant
Mean baseline body weight was 201 lbs and 206 lbs in the Metformin Hydrochloride Tablets and placebo arms, respectively. Mean change in body weight from baseline to week 29 was -1.4 lbs and -2.4 lbs in the Metformin Hydrochloride Tablets and placebo arms, respectively. A 29-week, double-blind, placebo-controlled study of Metformin Hydrochloride Tablets and glyburide, alone and in combination, was conducted in obese patients with type 2 diabetes mellitus who had failed to achieve adequate glycemic control while on maximum doses of glyburide (baseline FPG of approximately 250 mg/dL). Patients randomized to the combination arm started therapy with Metformin Hydrochloride Tablets 500 mg and glyburide 20 mg. At the end of each week of the first 4 weeks of the trial, these patients had their dosages of Metformin Hydrochloride Tablets increased by 500 mg if they had failed to reach target fasting plasma glucose. After week 4, such dosage adjustments were made monthly, although no patient was allowed to exceed Metformin Hydrochloride Tablets 2500 mg. Patients in the Metformin Hydrochloride Tablets only arm (metformin plus placebo) discontinued glyburide and followed the same titration schedule. Patients in the glyburide arm continued the same dose of glyburide. At the end of the trial, approximately 70% of the patients in the combination group were taking Metformin Hydrochloride Tablets 2000 mg/glyburide 20 mg or Metformin Hydrochloride Tablets 2500 mg/glyburide 20 mg. The results are displayed in Table 8.
Table 8: Mean Change in Fasting Plasma Glucose and HbA1c at Week 29 Comparing Metformin /Glyburide (Comb) vs Glyburide (Glyb) vs Metformin (Met): in Patients with Type 2 Diabetes Mellitus with Inadequate Glycemic Control on Glyburide
Comb
(n=213) Glyb
(n=209 )
GLU
(n=210) p-Values
Glyb vs
Comb Met vs
Comb Met vs
Glyb
Fasting Plasma Glucose (mg/dL)
Baseline
Change at FINAL VISIT 250.5
–63.5 247.5
13.7 253.9
–0.9 NS *
0.001 NS *
0.001 NS *
0.025
Hemoglobin A1c (%)
Baseline
Change at FINAL VISIT 8.8
–1.7 8.5
0.2 8.9
–0.4 NS *
0.001 NS *
0.001 0.007
0.001* Not statistically significant
Mean baseline body weight was 202 lbs, 203 lbs, and 204 lbs in the Metformin/glyburide, glyburide, and Metformin arms, respectively. Mean change in body weight from baseline to week 29 was 0.9 lbs, -0.7 lbs, and -8.4 lbs in the Metformin/ glyburide, glyburide, and Metformin arms, respectively.
Pediatric Clinical Studies
A double-blind, placebo-controlled study in pediatric patients aged 10 to 16 years with type 2 diabetes mellitus (mean FPG 182.2 mg/dL), treatment with Metformin Hydrochloride Tablets (up to 2000 mg/day) for up to 16 weeks (mean duration of treatment 11 weeks) was conducted. The results are displayed in Table 9.
Table 9: Mean Change in Fasting Plasma Glucose at Week 16 Comparing Metformin Hydrochloride Tablets vs Placebo in Pediatric Patients a with Type 2 Diabetes Mellitus
Metformin
Hydrochloride
Tablets
Placebo
p-Value
FPG (mg/dL)
Baseline
Change at FINAL VISIT (n=37)
162.4
–42.9 (n=36)
192.3
21.4 <0.001a Pediatric patients mean age 13.8 years (range 10-16 years)
Mean baseline body weight was 205 lbs and 189 lbs in the Metformin Hydrochloride Tablets and placebo arms, respectively. Mean change in body weight from baseline to week 16 was -3.3 lbs and -2.0 lbs in the Metformin Hydrochloride Tablets and placebo arms, respectively.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Metformin Hydrochloride Tablets, USP
500 mg Metformin Hydrochloride Tablets, USP 500 mg are blackberry flavored, white to off-white, round, biconvex, beveled edge film coated tablets, debossed with ‘SG’ on one side ‘105’ on other side.
NDC: 70518-3735-00
PACKAGING: 30 in 1 BLISTER PACK
Store at 20°to 25°C (68°to 77°F); excursions permitted to 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature.]
Repackaged and Distributed By:
Remedy Repack, Inc.
625 Kolter Dr. Suite #4 Indiana, PA 1-724-465-8762
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Lactic Acidosis:
Explain the risks of lactic acidosis, its symptoms, and conditions that predispose to its development. Advise patients to discontinue Metformin Hydrochloride Tablets immediately and to promptly notify their healthcare provider if unexplained hyperventilation, myalgias, malaise, unusual somnolence or other nonspecific symptoms occur. Counsel patients against excessive alcohol intake and inform patients about importance of regular testing of renal function while receiving Metformin Hydrochloride Tablets. Instruct patients to inform their doctor that they are taking Metformin Hydrochloride Tablets prior to any surgical or radiological procedure, as temporary discontinuation may be required [see Warnings and Precautions (5.1)].
Hypoglycemia
Inform patients that hypoglycemia may occur when Metformin Hydrochloride Tablets are coadministered with oral sulfonylureas and insulin. Explain to patients receiving concomitant therapy the risks of hypoglycemia, its symptoms and treatment, and conditions that predispose to its development [see Warnings and Precautions (5.3)].
Vitamin B 12 Deficiency:
Inform patients about importance of regular hematological parameters while receiving Metformin Hydrochloride Tablets [ see Warnings and Precautions (5.2)] .
Females of Reproductive Age:
Inform females that treatment with Metformin Hydrochloride Tablets may result in ovulation in some premenopausal anovulatory women which may lead to unintended pregnancy [ see Use in Specific Populations (8.3)].
All trademarks are the property of their respective owners.
Repackaged By / Distributed By: RemedyRepack Inc.
625 Kolter Drive, Indiana, PA 15701
(724) 465-8762
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
Metformin Hydrochloride Tablets, USP
(met for’ min hye’’ droe klor’ ide)
Read the Patient Information that comes with Metformin Hydrochloride Tablets before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your healthcare provider about your medical condition or treatment.
What is the most important information I should know about Metformin Hydrochloride Tablets?
Serious side effects can happen in people taking Metformin Hydrochloride Tablets, including:
Lactic Acidosis. Metformin hydrochloride, the medicine in Metformin Hydrochloride Tablets, can cause a rare, but serious, side effect called lactic acidosis (a build-up of lactic acid in the blood) that can cause death. Lactic acidosis is a medical emergency and must be treated in a hospital.
Stop taking Metformin Hydrochloride Tablets and call your healthcare provider right away if you get any of the following symptoms of lactic acidosis:
- feel very weak and tired
- have unusual (not normal) muscle pain
- have trouble breathing
- have unusual sleepiness or sleep longer than usual
- have unexplained stomach or intestinal problems with nausea and vomiting, or diarrhea
- feel cold, especially in your arms and legs
- feel dizzy or lightheaded
- have a slow or irregular heartbeat
You have a higher chance of getting lactic acidosis if you:
- have kidney problems. People whose kidneys are not working properly should not take Metformin Hydrochloride Tablets.
- have liver problems.
- have congestive heart failure that requires treatment with medicines.
- drink a lot of alcohol (very often or short-term “binge” drinking).
- get dehydrated (lose a large amount of body fluids). This can happen if you are sick with a fever, vomiting, or diarrhea. Dehydration can also happen when you sweat a lot with activity or exercise and do not drink enough fluids.
- have certain x-ray tests with injectable dyes or contrast agents.
- have surgery.
- have a heart attack, severe infection, or stroke.
- are 80 years of age or older and have not had your kidney function tested.
What are Metformin Hydrochloride Tablets?
- Metformin Hydrochloride Tablets are prescription medicines that contain metformin hydrochloride. Metformin Hydrochloride Tablets are used with diet and exercise to help control high blood sugar (hyperglycemia) in adults with type 2 diabetes.
- Metformin Hydrochloride Tablets are not for people with type 1 diabetes.
- Metformin Hydrochloride Tablets are not for people with diabetic ketoacidosis (increased ketones in your blood or urine).
Metformin Hydrochloride Tablets help control your blood sugar in a number of ways. These include helping your body respond better to the insulin it makes naturally, decreasing the amount of sugar your liver makes, and decreasing the amount of sugar your intestines absorb. Metformin Hydrochloride Tablets do not cause your body to make more insulin.
Who should not take Metformin Hydrochloride Tablets?
Some conditions increase your chance of getting lactic acidosis, or cause other problems if you take either of these medicines. Most of the conditions listed below can increase your chance of getting lactic acidosis.
Do not take Metformin Hydrochloride Tablets if you:
- have kidney problems
- are allergic to the metformin hydrochloride in Metformin Hydrochloride Tablets or any of the ingredients in Metformin Hydrochloride Tablets. See the end of this leaflet for a complete list of ingredients in Metformin Hydrochloride Tablets.
- are going to get an injection of dye or contrast agents for an x-ray procedure or if you are going to have surgery and not able to eat or drink much. In these situations, Metformin Hydrochloride Tablets will need to be stopped for a short time. Talk to your healthcare provider about when you should stop Metformin Hydrochloride Tablets and when you should start Metformin Hydrochloride Tablets again. See " “What is the most important information I should know about Metformin Hydrochloride Tablets?” "
What should I tell my healthcare provider before taking Metformin Hydrochloride Tablets?
Before taking Metformin Hydrochloride Tablets, tell your healthcare provider if you:
- have type 1 diabetes. Metformin Hydrochloride Tablets should not be used to treat people with type 1 diabetes.
- have a history or risk for diabetic ketoacidosis (high levels of certain acids, known as ketones, in the blood or urine). Metformin Hydrochloride Tablets should not be used for the treatment of diabetic ketoacidosis.
- have kidney problems.
- have liver problems.
- have heart problems, including congestive heart failure.
- are older than 80 years. If you are over 80 years old you should not take Metformin Hydrochloride Tablets unless your kidneys have been checked and they are normal.
- drink alcohol very often, or drink a lot of alcohol in short-term “binge” drinking
- are taking insulin.
- have any other medical conditions.
- are pregnant or plan to become pregnant. It is not known if Metformin Hydrochloride Tablets will harm your unborn baby. If you are pregnant, talk with your healthcare provider about the best way to control your blood sugar while you are pregnant.
- are breast-feeding or plan to breast-feed. It is not known if Metformin Hydrochloride Tablets passes into your breast milk. Talk with your healthcare provider about the best way to feed your baby while you take Metformin Hydrochloride Tablets.
Tell your healthcare provider about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
- Metformin Hydrochloride Tablets may affect the way other medicines work, and other medicines may affect how Metformin Hydrochloride Tablets works.
Can Metformin Hydrochloride Tablets be used in children?
Metformin Hydrochloride Tablets has been shown to effectively lower glucose levels in children (ages 10-16 years) with type 2 diabetes. Metformin Hydrochloride Tablets has not been studied in children younger than 10 years old. Metformin Hydrochloride Tablets has not been studied in combination with other oral glucose-control medicines or insulin in children. If you have any questions about the use of Metformin Hydrochloride Tablets in children, talk with your doctor or other healthcare provider.
How should I take Metformin Hydrochloride Tablets?
- Take Metformin Hydrochloride Tablets exactly as your healthcare provider tells you.
- Metformin Hydrochloride Tablets should be taken with meals to help lessen an upset stomach side effect.
- Swallow Metformin Hydrochloride Tablets whole
- You may sometimes pass a soft mass in your stools (bowel movement) that looks like Metformin Hydrochloride Tablets.
- When your body is under some types of stress, such as fever, trauma (such as a car accident), infection, or surgery, the amount of diabetes medicine that you need may change. Tell your healthcare provider right away if you have any of these problems.
- Your healthcare provider should do blood tests to check how well your kidneys are working before and during your treatment with Metformin Hydrochloride Tablets.
- Your healthcare provider will check your diabetes with regular blood tests, including your blood sugar levels and your hemoglobin A1C.
- Follow your healthcare provider’s instructions for treating blood sugar that is too low (hypoglycemia). Talk to your healthcare provider if low blood sugar is a problem for you. See " What are the possible side effects of Metformin Hydrochloride Tablets?” "
- Check your blood sugar as your healthcare provider tells you to.
- Stay on your prescribed diet and exercise program while taking Metformin Hydrochloride Tablets.
- If you miss a dose of Metformin Hydrochloride Tablets, take your next dose as prescribed unless your healthcare provider tells you differently. Do not take an extra dose the next day.
- If you take too much Metformin Hydrochloride Tablets, call your healthcare provider, local Poison Control Center, or go to the nearest hospital emergency room right away.
What should I avoid while taking Metformin Hydrochloride Tablets?
Do not drink a lot of alcoholic drinks while taking Metformin Hydrochloride Tablets. This means you should not binge drink for short periods, and you should not drink a lot of alcohol on a regular basis. Alcohol can increase the chance of getting lactic acidosis.
What are the side effects of Metformin Hydrochloride Tablets?
- Lactic acidosis. Metformin, the active ingredient in Metformin Hydrochloride Tablets, can cause a rare but serious condition called lactic acidosis (a buildup of an acid in the blood) that can cause death. Lactic acidosis is a medical emergency and must be treated in the hospital.
Call your doctor right away if you have any of the following symptoms, which could be signs of lactic acidosis:
- you feel cold in your hands or feet
- you feel dizzy or lightheaded
- you have a slow or irregular heartbeat
- you feel very weak or tired
- you have trouble breathing
- you feel sleepy or drowsy
- you have stomach pains, nausea or vomiting
Most people who have had lactic acidosis with metformin have other things that, combined with the metformin, led to the lactic acidosis. Tell your doctor if you have any of the following, because you have a higher chance for getting lactic acidosis with Metformin Hydrochloride Tablets if you:
- have severe kidney problems, or your kidneys are affected by certain x-ray tests that use injectable dye
- have liver problems
- drink alcohol very often, or drink a lot of alcohol in short-term “binge” drinking
- get dehydrated (lose a large amount of body fluids). This can happen if you are sick with a fever, vomiting, or diarrhea. Dehydration can also happen when you sweat a lot with activity or exercise and do not drink enough fluids
- have surgery
- have a heart attack, severe infection, or stroke
Common side effects of Metformin Hydrochloride Tablets include diarrhea, nausea, and upset stomach. These side effects generally go away after you take the medicine for a while. Taking your medicine with meals can help reduce these side effects. Tell your doctor if the side effects bother you a lot, last for more than a few weeks, come back after they’ve gone away, or start later in therapy. You may need a lower dose or need to stop taking the medicine for a short period or for good.
About 3 out of every 100 people who take Metformin Hydrochloride Tablets have an unpleasant metallic taste when they start taking the medicine. It lasts for a short time.
Metformin Hydrochloride Tablets rarely cause hypoglycemia (low blood sugar) by themselves. However, hypoglycemia can happen if you do not eat enough, if you drink alcohol, or if you take other medicines to lower blood sugar.
How should I store Metformin Hydrochloride Tablets?
Store Metformin Hydrochloride Tablets at 68°F to 77°F (20°C to 25°C).
Keep Metformin Hydrochloride Tablets and all medicines out of the reach of children.
General information about the use of Metformin Hydrochloride Tablets
If you have questions or problems, talk with your doctor or other healthcare provider. You can ask your doctor or pharmacist for the information about Metformin Hydrochloride Tablets that is written for healthcare professionals. Medicines are sometimes prescribed for purposes other than those listed in a patient information leaflet. Do not use Metformin Hydrochloride Tablets for a condition for which it was not prescribed. Do not share your medicine with other people.
What are the ingredients of Metformin Hydrochloride Tablets, USP?
Active ingredient of Metformin hydrochloride Tablets, USP: Metformin hydrochloride, USP
Inactive ingredients in each tablet of Metformin hydrochloride: pregelatinized starch (maize), povidone, crospovidone, magnesium stearate. In addition, the coating for the tablets contains hypromellose, polyethylene glycol, titanium dioxide and flavoring agent contains dextrose, ethyl alcohol, gum arabic, propylene glycol and silicon dioxide.
What is type 2 diabetes?
Type 2 diabetes is a condition in which your body does not make enough insulin, and the insulin that your body produces does not work as well as it should. Your body can also make too much sugar. When this happens, sugar (glucose) builds up in the blood. This can lead to serious medical problems.
The main goal of treating diabetes is to lower your blood sugar to a normal level.
High blood sugar can be lowered by diet and exercise, and by certain medicines when necessary.
Talk to your healthcare provider about how to prevent, recognize, and take care of low blood sugar (hypoglycemia), high blood sugar (hyperglycemia), and problems you have because of your diabetes.
All trademarks are the property of their respective owners.
Repackaged and Distributed By:
Remedy Repack, Inc.
625 Kolter Dr. Suite #4 Indiana, PA 1-724-465-8762
-
BOXED WARNING
(What is this?)
WARNING: LACTIC ACIDOSIS
WARNING: LACTIC ACIDOSIS
See full prescribing information for complete boxed warning.
Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. Symptoms included malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Laboratory abnormalities included elevated blood lactate levels, anion gap acidosis, increased lactate/pyruvate ratio; and metformin plasma levels generally >5 mcg/mL.( 5.1)
Risk factors include renal impairment, concomitant use of certain drugs, age >65 years old, radiological studies with contrast, surgery and other procedures, hypoxic states, excessive alcohol intake, and hepatic impairment. Steps to reduce the risk of and manage metformin associated lactic acidosis in these high risk groups are provided in the Full Prescribing Information. ( 5.1)
If lactic acidosis is suspected, discontinue Metformin Hydrochloride Tablets and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended. ( 5.1)Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin-associated lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metformin-associated lactic acidosis was characterized by elevated blood lactate levels (>5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/pyruvate ratio; and metformin plasma levels generally >5 mcg/mL [ see Warnings and Precautions (5.1)].
Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (e.g. carbonic anhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (e.g., acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high risk groups are provided [ see Dosage and Administration (2.3), Contraindications (4), Warnings and Precautions (5.1)].
If metformin-associated lactic acidosis is suspected, immediately discontinue metformin and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended [ see Warnings and Precautions (5.1)].
- 1 INDICATIONS AND USAGE
-
DOSAGE & ADMINISTRATION
Adult Dosage for Metformin Hydrochloride Tablets:
Starting dose: 500 mg orally twice a day or 850 mg once a day, with meals (2.1)
Increase the dose in increments of 500 mg weekly or 850 mg every 2 weeks, up to a maximum dose of 2550 mg per day, given in divided doses ( 2.1)
Doses above 2000 mg may be better tolerated given 3 times a day with meals ( 2.1)Pediatric Dosage for Metformin Hydrochloride Tablets:
Starting dose: 500 mg orally twice a day, with meals (2.2)
Increase dosage in increments of 500 mg weekly up to a maximum of 2000 mg per day, given in divided doses twice daily ( 2.2)Renal Impairment:
Prior to initiation, assess renal function with estimated glomerular filtration rate (eGFR) ( 2.3)
Do not use in patients with eGFR below 30 mL/minute/1.73 m 2 ( 2.3)
Initiation is not recommended in patients with eGFR between 30-45 mL/minute/1.73 m 2 ( 2.3)
Assess risk/benefit of continuing if eGFR falls below 45 mL/minute/1.73 m 2 ( 2.3)
Discontinue if eGFR falls below 30 mL/minute/1.73 m 2 ( 2.3)Discontinuation for Iodinated Contrast Imaging Procedures:
Metformin Hydrochloride Tablets may need to be discontinued at time of, or prior to, iodinated contrast imaging procedures ( 2.4)
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
Lactic Acidosis: See boxed warning.( 5.1)
Vitamin B 12 Deficiency: Metformin may lower vitamin B 12 levels. Measure hematological parameters annually and vitamin B 12 at 2 to 3 year intervals and manage any abnormalities. ( 5.2)
Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues: Increased risk of hypoglycemia when used in combination with insulin and/or an insulin secretagogue. Lower dose of insulin or insulin secretagogue may be required ( 5.3) -
7 DRUG INTERACTIONS
Table 3 presents clinically significant drug interactions with Metformin Hydrochloride tablets.
Table 3: Clinically Significant Drug Interactions with Metformin Hydrochloride Tablets
Carbonic Anhydrase Inhibitors
Clinical Impact:
Carbonic anhydrase inhibitors frequently cause a decrease in serum bicarbonate and induce non-anion gap, hyperchloremic metabolic acidosis. Concomitant use of these drugs with Metformin Hydrochloride may increase the risk for lactic acidosis.
Intervention:
Consider more frequent monitoring of these patients.
Examples:
Topiramate, zonisamide, acetazolamide or dichlorphenamide.
Drugs that Reduce Metformin Hydrochloride Clearance
Clinical Impact:
Concomitant use of drugs that interfere with common renal tubular transport systems involved in the renal elimination of metformin (e.g., organic cationic transporter-2 [OCT2] / multidrug and toxin extrusion [MATE] inhibitors) could increase systemic exposure to metformin and may increase the risk for lactic acidosis [ CLINICAL PHARMACOLOGY (12.3)].
Intervention:
Consider the benefits and risks of concomitant use with Metformin Hydrochloride.
Examples:
Ranolazine, vandetanib, dolutegravir, and cimetidine.
Alcohol
Clinical Impact:
Alcohol is known to potentiate the effect of metformin on lactate metabolism.
Intervention:
Warn patients against excessive alcohol intake while receiving Metformin Hydrochloride.
Insulin Secretagogues or Insulin
Clinical Impact:
Coadministration of Metformin Hydrochloride with an insulin secretagogue (e.g., sulfonylurea) or insulin may increase the risk of hypoglycemia.
Intervention:
Patients receiving an insulin secretagogue or insulin may require lower doses of the insulin secretagogue or insulin.
Drugs Affecting Glycemic Control
Clinical Impact:
Certain drugs tend to produce hyperglycemia and may lead to loss of glycemic control.
Intervention:
When such drugs are administered to a patient receiving Metformin Hydrochloride, observe the patient closely for loss of blood glucose control. When such drugs are withdrawn from a patient receiving Metformin Hydrochloride tablets, observe the patient closely for hypoglycemia.
Examples:
Thiazides and other diuretics, corticosteroids, phenothiazines, thyroid products, estrogens, oral contraceptives, phenytoin, nicotinic acid, sympathomimetics, calcium channel blockers, and isoniazid. - 8 USE IN SPECIFIC POPULATIONS
-
PRINCIPAL DISPLAY PANEL
DRUG: METFORMIN HYDROCHLORIDE
GENERIC: METFORMIN HYDROCHLORIDE
DOSAGE: TABLET
ADMINSTRATION: ORAL
NDC: 70518-3735-0
COLOR: white
FLAVOR: BLACKBERRY
SHAPE: ROUND
SCORE: No score
SIZE: 11 mm
IMPRINT: SG;105
PACKAGING: 30 in 1 BLISTER PACK
ACTIVE INGREDIENT(S):
- METFORMIN HYDROCHLORIDE 500mg in 1
INACTIVE INGREDIENT(S):
- STARCH, CORN
- POVIDONE, UNSPECIFIED
- CROSPOVIDONE, UNSPECIFIED
- MAGNESIUM STEARATE
- HYPROMELLOSE, UNSPECIFIED
- POLYETHYLENE GLYCOL, UNSPECIFIED
- TITANIUM DIOXIDE
- DEXTROSE, UNSPECIFIED FORM
- ALCOHOL
- ACACIA
- PROPYLENE GLYCOL
- SILICON DIOXIDE

-
INGREDIENTS AND APPEARANCE
METFORMIN HYDROCHLORIDE
metformin hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70518-3735(NDC:50228-105) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METFORMIN HYDROCHLORIDE (UNII: 786Z46389E) (METFORMIN - UNII:9100L32L2N) METFORMIN HYDROCHLORIDE 500 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) ALCOHOL (UNII: 3K9958V90M) ACACIA (UNII: 5C5403N26O) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color white (off-white) Score no score Shape ROUND Size 11mm Flavor BLACKBERRY Imprint Code SG;105 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70518-3735-0 30 in 1 BLISTER PACK; Type 0: Not a Combination Product 05/23/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203769 05/23/2023 Labeler - REMEDYREPACK INC. (829572556)

