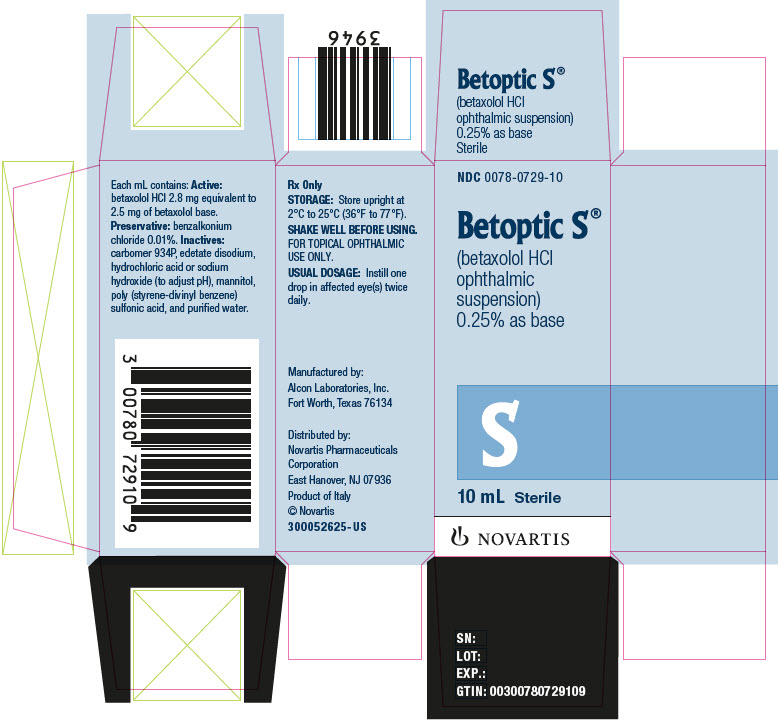
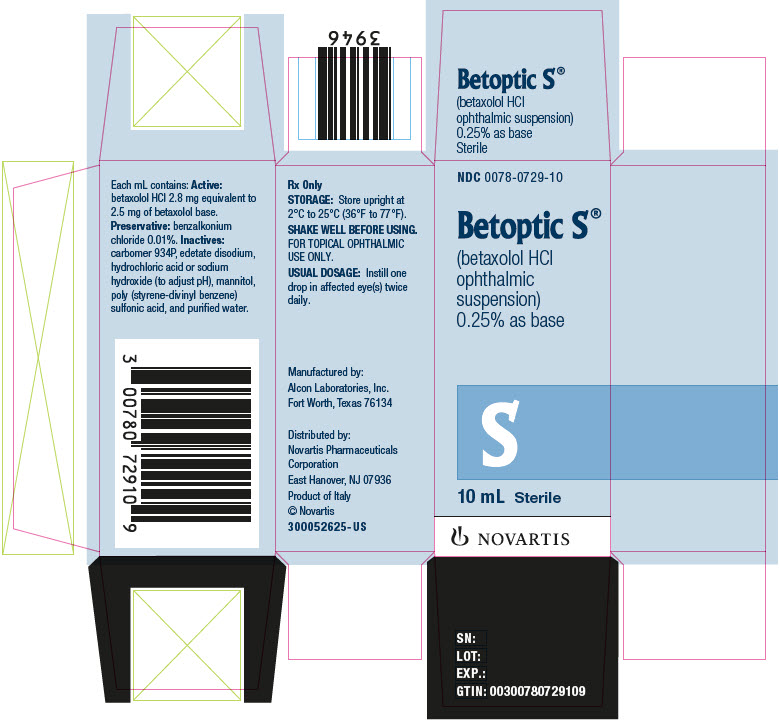
Label: BETOPTIC S- betaxolol hydrochloride suspension/ drops
- NDC Code(s): 0078-0729-10, 0078-0729-15
- Packager: Novartis Pharmaceuticals Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated July 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BETOPTIC S safely and effectively. See full prescribing information for BETOPTIC S.
BETOPTIC S® (betaxolol hydrochloride ophthalmic suspension), 0.25% as base, for topical ophthalmic use
Initial U.S. Approval: 1985INDICATIONS AND USAGE
BETOPTIC S is a beta-adrenergic receptor inhibitor indicated for the treatment of elevated intraocular pressure (IOP) in patients with chronic open-angle glaucoma or ocular hypertension. (1)
DOSAGE AND ADMINISTRATION
Instill one drop in the affected eye(s) twice daily. (2)
DOSAGE FORMS AND STRENGTHS
Ophthalmic suspension: 2.5 mg/mL of betaxolol as base (0.25%). (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Systemic Absorption: Same adverse reactions found with systemic administration of beta-adrenergic receptor inhibitors may occur with topical ophthalmic administration. (5.1)
- Cardiac Failure: Discontinue treatment at the first signs of cardiac failure. (5.2)
- Diabetes Mellitus: Beta-adrenergic receptor inhibitors may mask the signs and symptoms of acute hypoglycemia. Administer with caution in diabetic patients subject to hypoglycemia. (5.3)
- Thyrotoxicosis: Beta-adrenergic receptor inhibitors may mask certain clinical signs (e.g., tachycardia) or hyperthyroidism. (5.4)
ADVERSE REACTIONS
The most frequent adverse reaction is transient ocular discomfort. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2021
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Systemic Absorption
5.2 Cardiac Failure
5.3 Diabetes Mellitus
5.4 Thyrotoxicosis
5.5 Muscle Weakness
5.6 Surgical Anesthesia
5.7 Bronchospasm and Obstructive Pulmonary Disease
5.8 Atopy/Anaphylaxis
5.9 Angle-Closure Glaucoma
5.10 Vascular Insufficiency
5.11 Bacterial Keratitis
5.12 Choroidal Detachment
5.13 Contact Lens Wear
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Additional Potential Adverse Reactions Associated With Betaxolol
7 DRUG INTERACTIONS
7.1 Oral Beta-Adrenergic Receptor Inhibitors
7.2 Catecholamine-Depleting Drugs
7.3 Concomitant Adrenergic Psychotropic Drugs
7.4 Calcium Antagonists, Antiarrhythmics and Digitalis
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Instill one drop of BETOPTIC S in the affected eye(s) twice daily. Shake well before using.
BETOPTIC S may be used alone or in combination with other IOP lowering medications. Advise patients requiring concomitant topical ophthalmic medications to administer these at least 10 minutes before instilling BETOPTIC S.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Systemic Absorption
As with many topically applied ophthalmic drugs, this drug is absorbed systemically. The same adverse reactions found with systemic administration of beta-adrenergic receptor inhibitors may occur with topical administration. For example, severe respiratory reactions and cardiac reactions, including death due to bronchospasm in patients with asthma, and death due to cardiac failure, have been reported with topical application of beta-adrenergic receptor inhibitors.
5.2 Cardiac Failure
BETOPTIC S has been shown to have a minor effect on heart rate and blood pressure in clinical studies. Caution should be used in treating patients with a history of cardiac failure or heart block. Treatment with BETOPTIC S should be discontinued at the first signs of cardiac failure.
5.3 Diabetes Mellitus
Beta-adrenergic receptor inhibitors should be administered with caution in patients subject to hypoglycemia or to diabetic patients (especially those with labile diabetes) who are receiving insulin or oral hypoglycemic agents.
Beta-adrenergic receptor inhibitors may mask the signs and symptoms of acute hypoglycemia.
5.4 Thyrotoxicosis
Beta-adrenergic receptor inhibitors may mask certain clinical signs (e.g., tachycardia) of hyperthyroidism. Patients suspected of developing thyrotoxicosis should be managed carefully to avoid abrupt withdrawal of beta-adrenergic receptor inhibitors, which might precipitate a thyroid storm.
5.5 Muscle Weakness
Beta-adrenergic receptor inhibitors have been reported to potentiate muscle weakness consistent with certain myasthenic symptoms (e.g., diplopia, ptosis, and generalized weakness).
5.6 Surgical Anesthesia
The necessity or desirability of withdrawal of beta-adrenergic receptor inhibitors prior to major surgery is controversial. Beta-adrenergic receptor inhibitors impair the ability of the heart to respond to beta adrenergically mediated reflex stimuli. This may augment the risk of general anesthesia in surgical procedures. Some patients receiving beta-adrenergic receptor inhibitors have experienced protracted, severe hypotension during anesthesia. Difficulty in restarting and maintaining the heartbeat has also been reported. In patients undergoing elective surgery, consider gradual withdrawal of beta-adrenergic receptor inhibitors. If necessary during surgery, the effects of beta-adrenergic receptor inhibitors may be reversed by sufficient doses of adrenergic agonists.
5.7 Bronchospasm and Obstructive Pulmonary Disease
Caution should be exercised in the treatment of glaucoma patients with excessive restriction of pulmonary function. There have been reports of asthmatic attacks and pulmonary distress during betaxolol treatment. Although rechallenges of some such patients with ophthalmic betaxolol has not adversely affected pulmonary function test results, the possibility of adverse pulmonary effects in patients sensitive to beta-adrenergic receptor inhibitors cannot be ruled out.
5.8 Atopy/Anaphylaxis
While taking beta-adrenergic receptor inhibitors, patients with a history of atopy or a history of severe anaphylactic reaction to a variety of allergens may be more reactive to repeated accidental, diagnostic, or therapeutic challenge with such allergens. Such patients may be unresponsive to the usual doses of epinephrine used to treat anaphylactic reactions.
5.9 Angle-Closure Glaucoma
In patients with angle-closure glaucoma, the immediate treatment objective is to reopen the angle. This may require constricting the pupil. Betaxolol has little or no effect on the pupil and should not be used alone in the treatment of angle-closure glaucoma.
5.10 Vascular Insufficiency
Because of potential effects of beta-adrenergic receptor inhibitors on blood pressure and pulse, these inhibitors should be used with caution in patients with vascular insufficiency. If signs or symptoms suggesting reduced cerebral blood flow or Raynaud’s phenomenon develop following initiation of therapy with BETOPTIC S, alternative therapy should be considered.
5.11 Bacterial Keratitis
Bacterial keratitis may occur with use of multiple dose containers of topical ophthalmic products when these containers are inadvertently contaminated by patients who, in most cases, had a concurrent corneal disease or a disruption of the ocular epithelial surface. Instruct patients on appropriate instillation techniques [see Patient Counseling Information (17)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical trials, the most frequent adverse reaction associated with the use of BETOPTIC S has been transient ocular discomfort. The following other adverse reactions have been reported in small numbers of patients:
Ocular: blurred vision, corneal punctate keratitis, foreign body sensation, photophobia, tearing, itching, dryness of eyes, erythema, inflammation, discharge, ocular pain, decreased visual acuity, and crusty lashes.
Systemic Adverse Reactions Include:
Cardiovascular: Bradycardia, heart block, and congestive failure.
Pulmonary: Pulmonary distress characterized by dyspnea, bronchospasm, thickened bronchial secretions, asthma, and respiratory failure.
Central Nervous System: Insomnia, dizziness, vertigo, headaches, depression, lethargy, and increase in signs, and symptoms of myasthenia gravis.
Other: Hives, toxic epidermal necrolysis, hair loss and glossitis. Perversions of taste and smell have been reported.
In a 3-month, double-masked, active-controlled, multicenter study in pediatric patients, the adverse reaction profile of BETOPTIC S was comparable to that seen in adult patients.
-
7 DRUG INTERACTIONS
7.1 Oral Beta-Adrenergic Receptor Inhibitors
Patients who are receiving a beta-adrenergic receptor inhibitor orally and BETOPTIC S should be observed for a potential additive effect either on the IOP or on the known systemic effects of beta blockade.
7.2 Catecholamine-Depleting Drugs
Close observation of the patient is recommended when a beta-adrenergic receptor inhibitor is administered to patients receiving catecholamine-depleting drugs, such as reserpine, because of possible additive effects and the production of hypotension and/or bradycardia, which may result in vertigo, syncope, or postural hypotension.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies of BETOPTIC S administration in pregnant women to inform a drug-associated risk. There are limited data with the use of betaxolol eye drops in pregnant women. Epidemiological studies have not revealed malformative effects but show a risk for intrauterine growth retardation when beta-blockers are administered by the oral route.
In animal reproductive studies, no drug-induced maternal toxicity or teratogenicity was observed at clinically relevant doses (see Data).
Because animal reproductive studies are not always predictive of human response, BETOPTIC S should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
In the U.S. general population, the estimated background risk of major birth defects is 2% to 4% and of miscarriage is 15% to 20% of clinically recognized pregnancies.
Data
Animal Data
In a rat embryo-fetal development (EFD) study, oral administration of 4, 40, or 400 mg/kg/day betaxolol on gestational days 6 through 18, the period of organogenesis, resulted in marked embryo-fetal lethality, increased incidence of skeletal and visceral abnormalities, and decreased fetal weights at the maternally toxic dose of 400 mg/kg/day (7785 times higher than the maximum recommended human ophthalmic dose [MRHOD] of 0.0083 mg/kg/day, on a mg/m2 basis). The no-observed-adverse-effect-level (NOAEL) for maternal or embryo-fetal toxicity was 40 mg/kg/day (778 times higher than the MRHOD, on a mg/m2 basis). In a rabbit EFD study, oral administration of 1, 4, 12, and 36 mg/kg/day betaxolol on gestational days 6 through 18, the period of organogenesis, resulted in a marked increase in embryo-fetal lethality at 36 mg/kg/day (1400 times higher than the MRHOD, on a mg/m2 basis). No maternal toxicity was reported in this study.
In a perinatal and postnatal development study in rats, oral administration of 4, 32, and 256 mg/kg/day betaxolol during late gestation through lactation resulted in a marked decrease in offspring survival within 4 days postpartum at the highest dose (4982 times higher than the MRHOD, on a mg/m2 basis). In surviving F1 offspring, growth/development and reproductive function were also affected. The NOAEL was 32 mg/kg/day (623 times higher than the MRHOD, on a mg/m2 basis).
In a prenatal and postnatal development study in rats, an oral betaxolol dose of 150 mg/kg/day (2920 times higher than the MRHOD, on mg/m2 basis) administered throughout the entire gestation period and lactation, resulted in maternal and developmental toxicity in F1 offspring, including decreased offspring survival, body weight and growth/development and functional deficits in surviving offspring. No NOAEL for developmental or maternal toxicity was established in this study.
Oral administration of 4, 32, and 256 mg/kg/day betoxolol to rats prior to mating and through late gestation, or until weaning, produced embryo-fetal lethality, neonatal mortality, decreased mean fetal weight, growth/development and functional deficits in surviving offspring at 256 mg/kg/day (4982 times higher than the MRHOD, on a mg/m2 basis). At 32 mg/kg/day (623 higher than the MRHOD, on a mg/m2 basis), decreased mean fetal weight on Gestation Day 20, and pup weight at birth and on Day 4 postpartum were observed. At 4 mg/kg/day (78 times higher than the MRHOD, on a mg/m2 basis), a slight decrease in mean fetal weight was observed on Gestation Day 20.
8.2 Lactation
Risk Summary
Beta-blockers are excreted in breast milk following oral administration, having the potential to cause serious undesirable effects in the infant of the nursing mother. It is not known whether measurable levels of betaxolol would be present in maternal milk following topical ocular administration.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for BETOPTIC S, and any potential adverse effects on the breast-fed child from BETOPTIC S.
-
10 OVERDOSAGE
No information is available on overdosage in humans. The oral LD50 of the drug ranged from 350 to 920 mg/kg in mice and 860 to 1,050 mg/kg in rats. The symptoms which might be expected with an overdose of a systemically administered beta-adrenergic receptor inhibitor are bradycardia, hypotension, bronchospasm, and acute cardiac failure.
A topical overdose of BETOPTIC S may be flushed from the eye(s) with warm tap water. If overdose occurs, treatment should be symptomatic and supportive.
-
11 DESCRIPTION
BETOPTIC S contains betaxolol hydrochloride, a cardioselective beta-adrenergic receptor inhibitor, in a sterile resin suspension formulation. Betaxolol hydrochloride is a white, crystalline powder, with a molecular weight of 343.89 g/mol. The chemical structure is presented below.
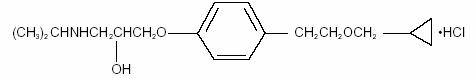
Empirical Formula: C18H29NO3•HCl
Chemical Name:
(±)-1-[p-[2-(cyclopropylmethoxy) ethyl]phenoxy]-3-(isopropylamino)-2-propanol hydrochloride.Each mL of BETOPTIC S contains: Active: betaxolol HCl 2.8 mg equivalent to 2.5 mg of betaxolol base. Preservative: benzalkonium chloride 0.01%. Inactives: carbomer 934P, edetate disodium, hydrochloric acid or sodium hydroxide (to adjust pH), mannitol, poly (styrene-divinyl benzene) sulfonic acid, and purified water.
BETOPTIC S has pH of approximately 7.6 and an osmolality of approximately 290 mOsmol/kg.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Betaxolol HCl, a cardioselective (beta-1-adrenergic) receptor inhibitor, does not have significant membrane-stabilizing (local anesthetic) activity and is devoid of intrinsic sympathomimetic action. Orally administered beta-adrenergic receptor inhibitors reduce cardiac output in healthy subjects and patients with heart disease. In patients with severe impairment of myocardial function, beta-adrenergic receptor antagonists may inhibit the sympathetic stimulatory effect necessary to maintain adequate cardiac function.
When instilled in the eye, BETOPTIC S has the action of reducing elevated IOP, whether or not accompanied by glaucoma. Ophthalmic betaxolol has minimal effect on pulmonary and cardiovascular parameters.
Elevated IOP presents a major risk factor in glaucomatous field loss. The higher the level of IOP, the greater the likelihood of optic nerve damage and visual field loss. Betaxolol has the action of reducing elevated as well as normal IOP and the mechanism of ocular hypotensive action appears to be a reduction of aqueous production as demonstrated by tonography and aqueous fluorophotometry.
12.2 Pharmacodynamics
The onset of action with betaxolol can generally be noted within 30 minutes and the maximum effect can usually be detected 2 hours after topical administration. A single dose provides a 12-hour reduction in IOP. In some patients, the IOP lowering responses to BETOPTIC S may require a few weeks to stabilize. As with any new medication, careful monitoring of patients is advised.
Ophthalmic betaxolol solution at 1% (one drop in each eye) was compared to placebo in a crossover study challenging nine patients with reactive airway disease. Betaxolol HCl had no significant effect on pulmonary function as measured by forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), FEV1/FVC, and was not significantly different from placebo. The action of isoproterenol, a beta stimulant, administered at the end of the study was not inhibited by ophthalmic betaxolol.
No evidence of cardiovascular beta-adrenergic blockade during exercise was observed with betaxolol in a double-masked, crossover study in 24 normal subjects comparing ophthalmic betaxolol and placebo for effects on blood pressure and heart rate.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In 2-year carcinogenicity studies, betaxolol HCl demonstrated no carcinogenic effect at doses up to 60 mg/kg/day in mice and 48 mg/kg/day in rats (584 and 934 times higher than the MRHOD, on a mg/m2 basis, respectively).
Mutagenesis
In a variety of in vitro and in vivo bacterial and mammalian cell assays, betaxolol HCl was not mutagenic.
Impairment of Fertility
Betaxolol did not adversely affect fertility or mating performance of male or female rats at doses up to 256 mg/kg/day (4982 times higher than the MRHOD, on a mg/m2 basis).
- 14 CLINICAL STUDIES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
BETOPTIC S (betaxolol hydrochloride ophthalmic suspension), 0.25% is supplied as follows: 10 mL and 15 mL in plastic ophthalmic dispensers. Tamper evidence is provided with a shrink band around the closure and neck area of the package.
10 mL NDC 0078-0729-10
15 mL NDC 0078-0729-15
Storage and Handling
Store upright at 2°C to 25°C (36°F to 77°F).
Shake well before using.
After opening, BETOPTIC S can be used until the expiration date on the bottle.
-
17 PATIENT COUNSELING INFORMATION
Avoiding Contamination of the Product
Instruct patients to avoid allowing the tip of the dispensing container to contact the eye(s) or surrounding structures. Also instruct patients that ocular solutions, if handled improperly, could become contaminated by common bacteria known to cause ocular infections. Serious damage to the eye(s) and subsequent loss of vision may result from using contaminated solutions.
Intercurrent Ocular Conditions
Advise patients that if they have ocular surgery or develop an intercurrent ocular condition (e.g., trauma or infection), they should immediately seek their physician’s advice concerning the continued use of the present multi-dose container.
Concomitant Topical Ocular Therapy
Advise patients requiring concomitant topical ophthalmic medications to administer these at least 10 minutes before instilling BETOPTIC S.
Temporary Blurred Vision
Vision may be temporarily blurred following dosing with BETOPTIC S. Care should be exercised in operating machinery or driving a motor vehicle.
Contact Lens Wear
The preservative in BETOPTIC S, benzalkonium chloride, may be absorbed by soft contact lenses. Remove contact lenses during instillation of BETOPIC S. Contact lenses may be reinserted 15 minutes after instillation.
Distributed by:
Novartis Pharmaceuticals Corporation
East Hanover, New Jersey 07936© Novartis
T2021-95
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BETOPTIC S
betaxolol hydrochloride suspension/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0078-0729 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Betaxolol Hydrochloride (UNII: 6X97D2XT0O) (Betaxolol - UNII:O0ZR1R6RZ2) Betaxolol Hydrochloride 2.8 mg in 1 mL Inactive Ingredients Ingredient Name Strength Mannitol (UNII: 3OWL53L36A) Edetate Disodium (UNII: 7FLD91C86K) Hydrochloric Acid (UNII: QTT17582CB) Sodium Hydroxide (UNII: 55X04QC32I) Benzalkonium Chloride (UNII: F5UM2KM3W7) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0078-0729-10 1 in 1 CARTON 08/03/2022 1 10 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:0078-0729-15 1 in 1 CARTON 08/03/2022 2 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019845 01/15/1996 Labeler - Novartis Pharmaceuticals Corporation (002147023)