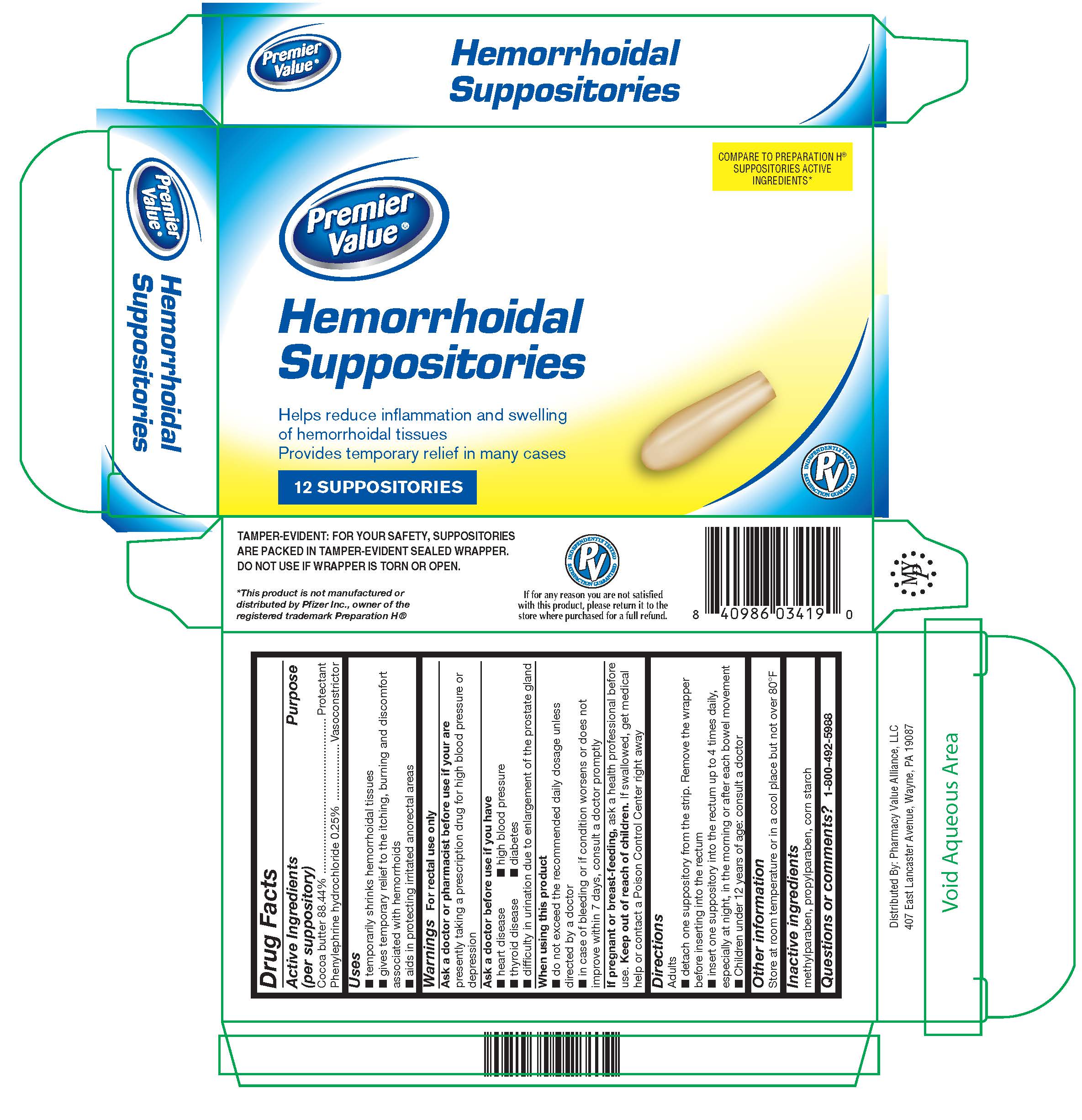
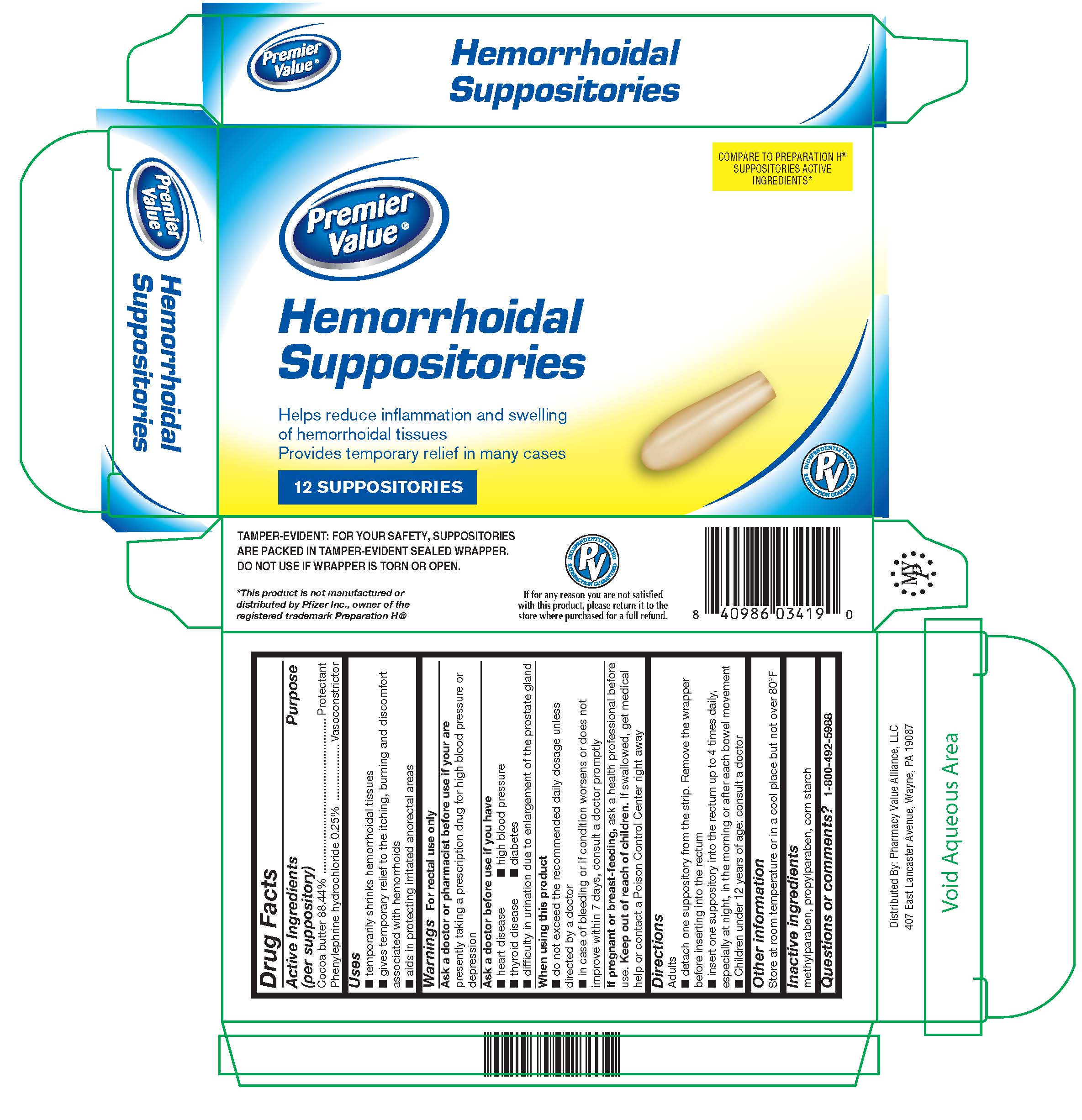
Label: HEMORRHOIDAL- cocoa butter, phenylephrine hcl suppository
- NDC Code(s): 68016-322-12, 68016-322-24
- Packager: Pharmacy Value Alliance LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients (per suppository)
- Purpose
- Uses
-
Warnings
For rectal use only
Ask a doctor or pharmacist before use if you are
presently taking a prescription drug for high blood pressure or depression
Ask a doctor before use if you have
- heart disease
- thyroid disease
- difficulty in urination due to enlargement of the prostate gland
- high blood pressure
- diabetes
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HEMORRHOIDAL
cocoa butter, phenylephrine hcl suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68016-322 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COCOA BUTTER (UNII: 512OYT1CRR) (COCOA BUTTER - UNII:512OYT1CRR) COCOA BUTTER 2.39 g PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 6.75 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68016-322-12 2 in 1 CARTON 08/16/2019 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:68016-322-24 4 in 1 CARTON 08/16/2019 2 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 08/16/2019 Labeler - Pharmacy Value Alliance LLC (101668460) Registrant - DSC Laboratories Inc. (097807374) Establishment Name Address ID/FEI Business Operations DSC Laboratories Inc. 097807374 manufacture(68016-322)