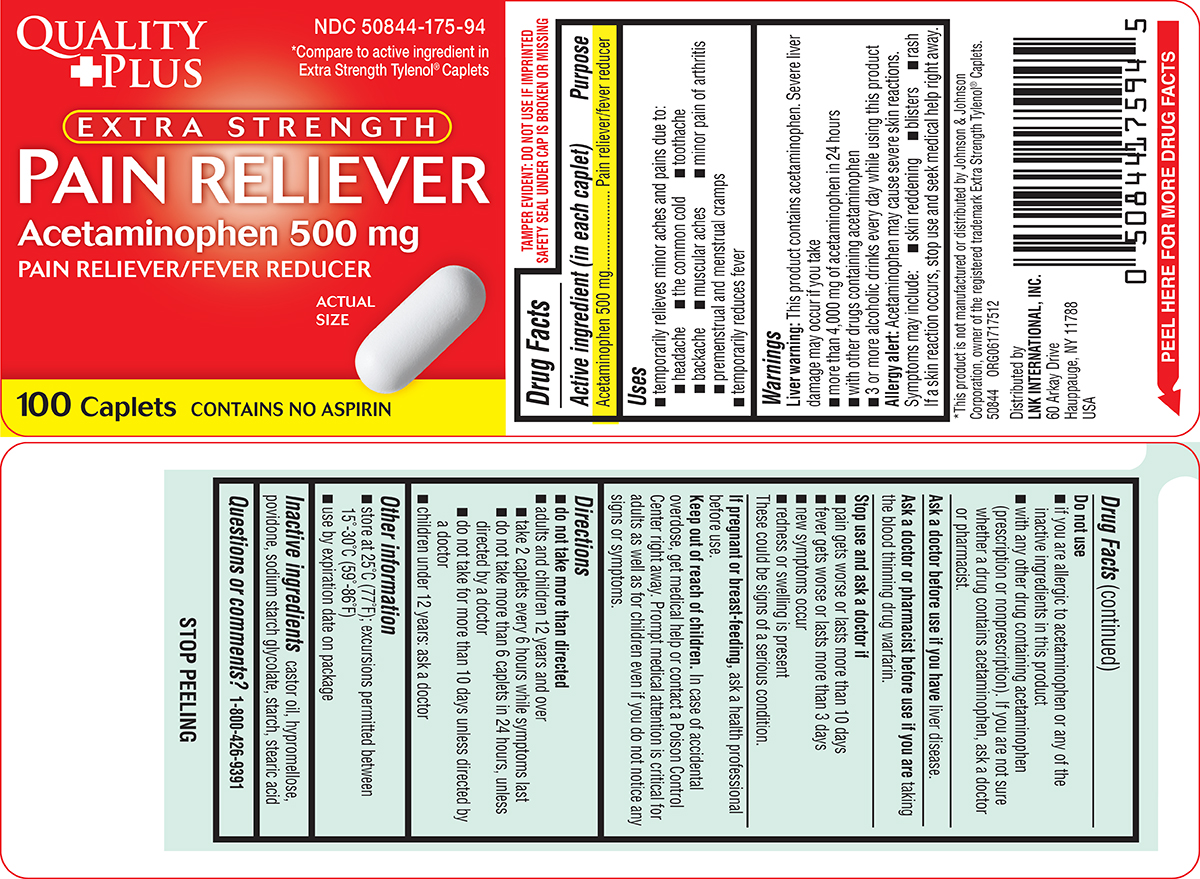
Label: PAIN RELIEVER EXTRA STRENGTH- acetaminophen tablet, film coated
- NDC Code(s): 50844-175-08, 50844-175-10, 50844-175-12, 50844-175-94
- Packager: L.N.K. International, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each caplet)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
QUALITY
+PLUSNDC 50844-175-94
*Compare to active ingredient in
Extra Strength Tylenol® CapletsEXTRA STRENGTH
PAIN RELIEVER
Acetaminophen 500 mg
PAIN RELIEVER/FEVER REDUCERACTUAL
SIZE100 Caplets
CONTAINS NO ASPIRIN
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING*This product is not manufactured or distributed by Johnson & Johnson
Corporation, owner of the registered trademark Extra Strength Tylenol® Caplets.50844 ORG061717512
Distributed by
LNK INTERNATIONAL, INC.
60 Arkay Drive
Hauppauge, NY 11788
USA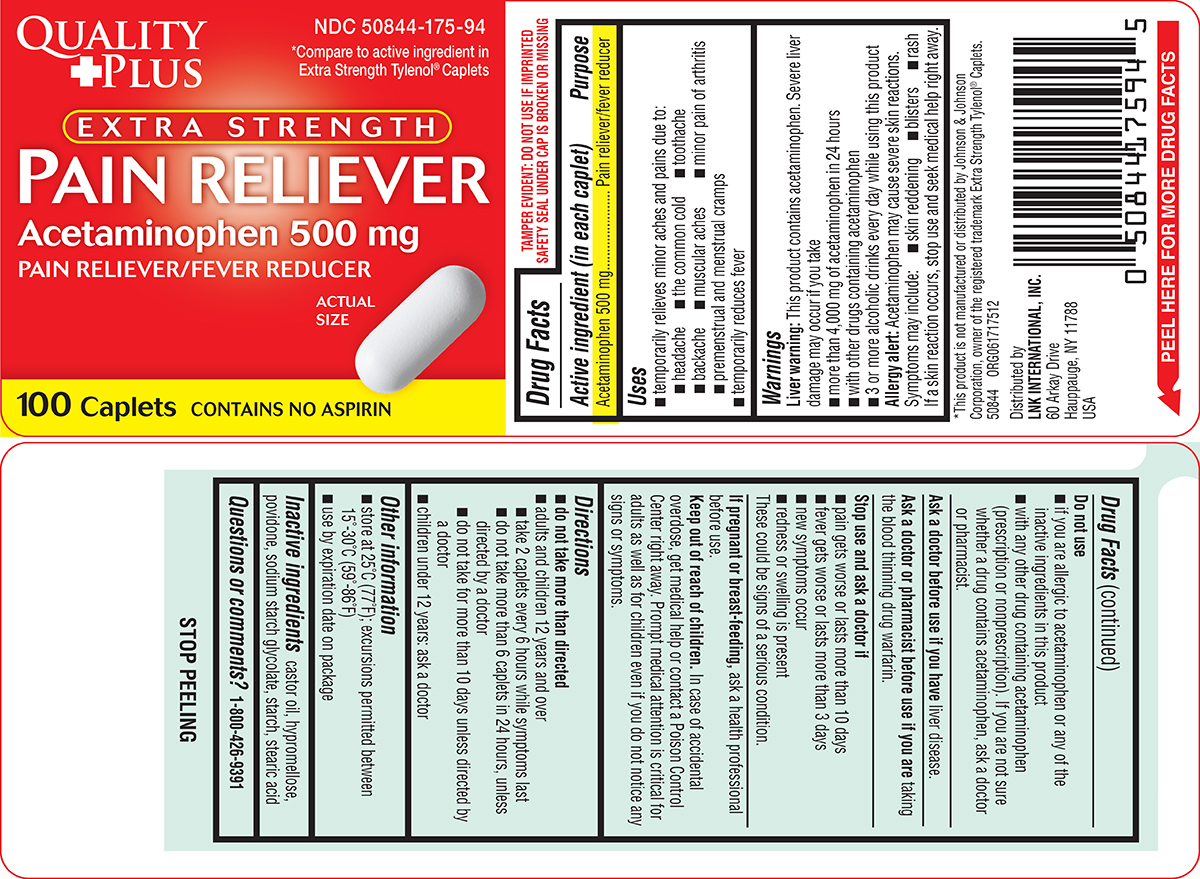
Quality Plus 44-175
-
INGREDIENTS AND APPEARANCE
PAIN RELIEVER EXTRA STRENGTH
acetaminophen tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50844-175 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg Inactive Ingredients Ingredient Name Strength CASTOR OIL (UNII: D5340Y2I9G) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white Score no score Shape OVAL Size 17mm Flavor Imprint Code 44;175 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50844-175-94 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/02/1993 2 NDC:50844-175-08 1 in 1 CARTON 04/02/1993 04/19/2022 2 24 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 3 NDC:50844-175-12 1 in 1 CARTON 04/02/1993 04/19/2022 3 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 4 NDC:50844-175-10 1 in 1 CARTON 04/02/1993 04/19/2022 4 40 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 04/02/1993 Labeler - L.N.K. International, Inc. (038154464) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(50844-175) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(50844-175) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 manufacture(50844-175) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 manufacture(50844-175) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(50844-175) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117597853 pack(50844-175)