Label: CRESEMBA- isavuconazonium sulfate capsule
CRESEMBA- isavuconazonium sulfate injection, powder, lyophilized, for solution
- NDC Code(s): 0469-0420-01, 0469-0520-02, 0469-2860-35
- Packager: Astellas Pharma US, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 7, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CRESEMBA® safely and effectively. See full prescribing information for CRESEMBA®. CRESEMBA® (isavuconazonium sulfate) capsules, for ...These highlights do not include all the information needed to use CRESEMBA® safely and effectively. See full prescribing information for CRESEMBA®.
CRESEMBA® (isavuconazonium sulfate) capsules, for oral use
CRESEMBA® (isavuconazonium sulfate) for injection, for intravenous use
Initial U.S. Approval: 2015INDICATIONS AND USAGE
CRESEMBA® is an azole antifungal indicated for the treatment of:
Invasive aspergillosis (1.1) and Invasive mucormycosis (1.2) as follows:
- •
- CRESEMBA for injection: adults and pediatric patients 1 year of age and older
- •
- CRESEMBA capsules: adults and pediatric patients 6 years of age and older who weigh 16 kilograms (kg) and greater
DOSAGE AND ADMINISTRATION
Important Administration Instructions:
- •
- CRESEMBA for injection is intended for use in patients who are 1 year of age and older (2.1, 2.3).
- •
- CRESEMBA for injection via nasogastric (NG) tube administration is intended for use by patients who are 6 years of age and older and weighing 16 kg and greater. (2.1, 2.6).
- •
- CRESEMBA for injection must be administered through an in-line filter over a minimum of 1 hour. (2.1, 2.5).
- •
- CRESEMBA capsules are intended for use in patients who are 6 years of age and older and weighing 16 kg and greater (2.1, 2.3).
- •
- CRESEMBA capsules can be taken with or without food. (2.1).
Recommended Dosage in Adult Patients (2.2):
Recommended Dosage for CRESEMBA in Adult Patients (2.2)
1Start maintenance doses 12 to 24 hours after the last loading dose Dosage Form
Loading Dose
Maintenance Dose1
CRESEMBA for Injection, 372 mg/vial
372 mg of isavuconazonium sulfate per vial
One reconstituted vial (372 mg)
intravenously
every 8 hours for 6 doses (48 hours)
One reconstituted vial (372 mg)
intravenously
once daily
CRESEMBA Capsules, 186 mg
186 mg of isavuconazonium sulfate per capsule
Two 186 mg capsules (372 mg)
orally
every 8 hours for 6 doses (48 hours)
Two 186 mg capsules (372 mg)
orally
once daily
CRESEMBA Capsules, 74.5 mg
74.5 mg of isavuconazonium sulfate per capsule
Five 74.5 mg capsules (372 mg)
orally
every 8 hours for 6 doses (48 hours)
Five 74.5 mg capsules (372 mg)
orally
once daily
Recommended Dosage in Pediatric Patients (2.3):
- •
- The maximum of any individual loading or daily maintenance dose to be administered to any pediatric patient is 372 mg of CRESEMBA. (2.3)
Recommended Dosage for CRESEMBA in Pediatric Patients (2.3) Dosage Form
Age Body Weight (kg) Loading Dose Maintenance Dose1 1Start maintenance doses 12 to 24 hours after the last loading dose CRESEMBA for Injection, 372 mg/vial
372 mg of isavuconazonium sulfate per vial
1 to less than 3 years of age
less than 18 kg
15 mg/kg
intravenously
every 8 hours for 6 doses (48 hours)
15 mg/kg
intravenously
once daily
3 to less than 18 years of age
less than 37 kg
10 mg/kg
intravenously
every 8 hours for 6 doses (48 hours)
10 mg/kg
intravenously
once daily
greater than or equal to 37 kg
One reconstituted vial (372 mg)
intravenously
every 8 hours for 6 doses (48 hours)
One reconstituted vial (372 mg)
intravenously
once daily
CRESEMBA Capsules, 74.5 mg
74.5 mg of isavuconazonium sulfate per capsule
6 to less than 18 years of age
16 kg to less than 18 kg
Two capsules (149 mg)
orally
every 8 hours for 6 doses (48 hours)
Two capsules (149 mg)
orally
once daily
18 kg to less than 25 kg
Three capsules (223.5 mg)
orally
every 8 hours for 6 doses (48 hours)
Three capsules (223.5 mg)
orally
once daily
25 kg to less than 32kg
Four capsules (298 mg)
orally
every 8 hours for 6 doses (48 hours)
Four capsules (298 mg)
orally
once daily
greater than or equal to 32 kg
Five 74.5 mg capsules (372 mg)
orally
every 8 hours for 6 doses (48 hours)
Five 74.5 mg capsules (372 mg)
orally
once daily
DOSAGE FORMS AND STRENGTHS
- •
- CRESEMBA for injection is supplied in a single-dose vial as a sterile lyophilized powder containing 372 mg of isavuconazonium sulfate (equivalent to 200 mg of isavuconazole). (3)
- •
- CRESEMBA capsules: 74.5 mg of isavuconazonium sulfate (equivalent to 40 mg of isavuconazole), 186 mg of isavuconazonium sulfate (equivalent to 100 mg of isavuconazole). (3)
CONTRAINDICATIONS
- •
- Hypersensitivity to CRESEMBA. (4)
- •
- Coadministration with strong CYP3A4 inhibitors, such as ketoconazole or high-dose ritonavir. (4, 7)
- •
- Coadministration with strong CYP3A4 inducers, such as rifampin, carbamazepine, St. John’s wort, or long acting barbiturates. (4, 7)
- •
- Use in patients with familial short QT syndrome. (4)
WARNINGS AND PRECAUTIONS
- •
- Hepatic Adverse Drug Reactions: Serious hepatic reactions have been reported. Evaluate liver-related laboratory tests at the start and during the course of CRESEMBA therapy. (5.1)
- •
- Infusion-related reactions were reported during intravenous administration of CRESEMBA. Discontinue the infusion if these reactions occur. (5.2)
- •
- Hypersensitivity Reactions: Anaphylactic reactions, with fatal outcome, have been reported during treatment with CRESEMBA. Serious skin reactions, such as Stevens-Johnson syndrome, have been reported during treatment with other azole antifungal agents. Discontinue CRESEMBA if anaphylactic or serious skin reactions occur, and initiate supportive treatment as needed. (5.3)
- •
- Embryo-Fetal Toxicity: CRESEMBA may cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to the fetus. Advise females of reproductive potential to use an effective method of contraception. (5.4, 8.1, 8.3)
- •
- Drug Interactions: Review patient’s concomitant medications. Several drugs may significantly alter isavuconazole concentrations. Isavuconazole may alter concentrations of several drugs. (5.5, 7, 12.3)
- •
- Drug Particulates: Intravenous formulation may form insoluble particulates following reconstitution. Administer CRESEMBA through an in-line filter. (2.4, 5.6)
ADVERSE REACTIONS
- •
- Adult Patients: The most frequent adverse reactions in adult patients were nausea, vomiting, diarrhea, headache, elevated liver chemistry tests, hypokalemia, constipation, dyspnea, cough, peripheral edema, and back pain. (6.1)
- •
-
Pediatric Patients: The most frequent adverse reactions in pediatric patients were diarrhea, abdominal pain, vomiting, elevated liver chemistry tests, rash, nausea, pruritus, and headache. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Astellas Pharma US, Inc. at 1-800-727-7003 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- •
- CYP3A4 inhibitors or inducers may alter the plasma concentrations of isavuconazole. (7)
- •
- Appropriate therapeutic drug monitoring and dose adjustment of immunosuppressants (i.e., tacrolimus, sirolimus, and cyclosporine) may be necessary when coadministered with CRESEMBA. (7)
- •
- Drugs with a narrow therapeutic window that are P-gp substrates, such as digoxin, may require dose adjustment when administered concomitantly with CRESEMBA. (7)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2025
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Invasive Aspergillosis
1.2 Invasive Mucormycosis
1.3 Usage
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions for CRESEMBA
2.2 Recommended Dosage and Administration in Adult Patients
2.3 Recommended Dosage and Administration in Pediatric Patients
2.4 Reconstitution Instructions for the CRESEMBA for Injection Formulation
2.5 Dilution and Preparation Instructions for the Intravenous Administration of the CRESEMBA for Injection Formulation
2.6 Preparation Instructions for the Nasogastric Tube Administration of the CRESEMBA for Injection Formulation
2.7 Compatibility for the Injection Formulation
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hepatic Adverse Drug Reactions
5.2 Infusion-Related Reactions
5.3 Hypersensitivity Reactions
5.4 Embryo-Fetal Toxicity
5.5 Drug Interactions
5.6 Drug Particulates
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Treatment of Invasive Aspergillosis
14.2 Treatment of Invasive Mucormycosis
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE1.1 Invasive Aspergillosis - CRESEMBA® is indicated for the treatment of invasive aspergillosis as follows: CRESEMBA for injection: adults and pediatric patients 1 year of age and older ...
1.1 Invasive Aspergillosis
CRESEMBA® is indicated for the treatment of invasive aspergillosis as follows:
CRESEMBA for injection: adults and pediatric patients 1 year of age and older [see Clinical Studies (14.1) and Clinical Pharmacology (12.4)]
CRESEMBA capsules: adults and pediatric patients 6 years of age and older who weigh 16 kilograms (kg) and greater [see Dosage and Administration (2.3) Clinical Studies (14.1) and Clinical Pharmacology (12.4)]
1.2 Invasive Mucormycosis
CRESEMBA is indicated for the treatment of invasive mucormycosis as follows:
CRESEMBA for injection: adults and pediatric patients 1 year of age and older [see Clinical Studies (14.1) and Clinical Pharmacology (12.3, 12.4)]
CRESEMBA capsules: adults and pediatric patients 6 years of age and older who weigh 16 kg and greater [see Dosage and Administration (2.3)], Clinical Studies (14.1) and Clinical Pharmacology (12.3, 12.4)]
Close1.3 Usage
Specimens for fungal culture and other relevant laboratory studies (including histopathology) to isolate and identify causative organism(s) should be obtained prior to initiating antifungal therapy. Therapy may be instituted before the results of the cultures and other laboratory studies are known. However, once these results become available, antifungal therapy should be adjusted accordingly.
-
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Instructions for CRESEMBA - CRESEMBA for Injection - • CRESEMBA for injection is intended for use in patients who are 1 year of age and older [see Dosage and ...
2.1 Important Administration Instructions for CRESEMBA
CRESEMBA for Injection
- •
- CRESEMBA for injection is intended for use in patients who are 1 year of age and older [see Dosage and Administration (2.2 and 2.3)].
- •
- Intravenous formulation must be administered via an infusion set with an in-line filter (pore size 0.2 to 1.2 micron).
- •
- Infuse the intravenous formulation over a minimum of 1 hour in 250 mL of a compatible diluent, to reduce the risk for infusion-related reactions. Do not administer as an intravenous bolus injection.
- •
- Do not infuse CRESEMBA with other intravenous medications.
- •
- Flush intravenous lines with 0.9% sodium chloride injection, USP or 5% dextrose injection, USP prior to and after infusion of CRESEMBA.
- •
- After dilution of the intravenous formulation, avoid unnecessary vibration or vigorous shaking of the solution. Do not use a pneumatic transport system.
Nasogastric Tube Administration of CRESEMBA for Injection
- •
- CRESEMBA for injection via nasogastric (NG) tube administration is intended for use by patients who are 6 years of age and older and weighing 16 kg and greater [see Dosage and Administration (2.2, 2.3 and 2.6].
CRESEMBA Capsules
- •
- CRESEMBA capsules are intended for use in patients who are 6 years of age and older and weighing 16 kg and greater [see Dosage and Administration (2.2 and 2.3)].
- •
- Switching between the intravenous and oral formulations of CRESEMBA is acceptable as bioequivalence has been demonstrated. Loading dose is not required when switching between formulations.
- •
- With oral administration, swallow CRESEMBA capsules whole. Do not chew, crush, dissolve, or open the capsules. CRESEMBA capsules can be taken with or without food.
2.2 Recommended Dosage and Administration in Adult Patients
Recommended dosage and administration of CRESEMBA for injection and capsules in adult patients is described in Table 1 below. CRESEMBA (isavuconazonium sulfate) is the prodrug of isavuconazole, an azole antifungal drug.
Table 1. Recommended Dosage and Administration for CRESEMBA in Adult Patients Dosage Form Loading Dose Maintenance Dose* CRESEMBA for Injection, 372 mg/vial
372 mg† of isavuconazonium sulfate per vial
One reconstituted vial (372 mg†)
intravenously
every 8 hours for 6 doses (48 hours)
One reconstituted vial (372 mg†)
intravenously
once daily
CRESEMBA Capsules,
186 mg
186 mg‡ of isavuconazonium sulfate per capsule
Two 186 mg capsules (372 mg†)
orally
every 8 hours for 6 doses (48 hours)
Two 186 mg capsules (372 mg†)
orally
once daily
CRESEMBA Capsules, 74.5 mg
74.5 mg§ of isavuconazonium sulfate per capsule
Five 74.5 mg capsules (372 mg†)
orally
every 8 hours for 6 doses (48 hours)
Five 74.5 mg capsules (372 mg†)
orally
once daily
2.3 Recommended Dosage and Administration in Pediatric Patients
Recommended dosage and administration of CRESEMBA for injection and CRESEMBA capsules in pediatric patients is described in Table 2 below [see Clinical Pharmacology (12.3)]. The maximum of any individual loading or daily maintenance dose to be administered to any pediatric patient is 372 mg of CRESEMBA.
Table 2. Recommended Dosage and Administration for CRESEMBA in Pediatric Patients Dosage Form Age Body Weight (kg) Loading Dose Maintenance Dose* - *
- Start maintenance doses 12 to 24 hours after the last loading dose
- †
- 372 mg of isavuconazonium sulfate is equivalent to 200 mg of isavuconazole
- ‡
- 74.5 mg of isavuconazonium sulfate is equivalent to 40 mg of isavuconazole
- §
- Five 74.5 mg CRESEMBA capsules are equivalent to two 186 mg CRESEMBA capsules
CRESEMBA for Injection,
372 mg/vial
372 mg† of isavuconazonium sulfate per vial
1 year to less than 3 years of age
less than 18 kg
15 mg/kg
intravenously
every 8 hours for 6 doses (48 hours)
15 mg/kg
intravenously
once daily
3 years to less than 18 years of age
less than 37 kg
10 mg/kg
intravenously
every 8 hours for 6 doses (48 hours)
10 mg/kg
intravenously
once daily
greater than or equal to 37 kg
One reconstituted vial (372 mg†)
intravenously
every 8 hours for 6 doses (48 hours)
One reconstituted vial (372 mg†)
intravenously
once daily
CRESEMBA Capsules,
74.5 mg
74.5 mg‡ of isavuconazonium sulfate per capsule
6 to less than 18 years of age
16 kg to less than 18 kg
Two capsules (149 mg)
orally
every 8 hours for 6 doses (48 hours)
Two capsules (149 mg)
orally
once daily
18 kg to less than 25 kg
Three capsules (223.5 mg)
orally
every 8 hours for 6 doses (48 hours)
Three capsules (223.5 mg)
orally
once daily
25 kg to less than 32 kg
Four capsules (298 mg)
orally
every 8 hours for 6 doses (48 hours)
Four capsules (298 mg)
orally
once daily
greater than or equal to 32 kg
Five capsules§ (372 mg)
orally
every 8 hours for 6 doses (48 hours)
Five capsules§ (372 mg)
orally
once daily
2.4 Reconstitution Instructions for the CRESEMBA for Injection Formulation
Aseptic technique must be strictly observed in all handling since no preservative or bacteriostatic agent is present in CRESEMBA or in the materials specified for reconstitution. CRESEMBA is water soluble, preservative-free, sterile, and nonpyrogenic.
- •
- Reconstitute one vial of CRESEMBA by adding 5 mL water for injection, USP to the vial. The resultant solution will be 74.4 mg/mL of isavuconazonium sulfate.
- •
- Gently shake to dissolve the powder completely.
- •
- Visually inspect the reconstituted solution for particulate matter and discoloration. Reconstituted CRESEMBA should be clear and free of visible particulate.
- •
- The reconstituted solution may be stored between 5°C to 25°C (41°F to 77°F) for a maximum of 1 hour prior to preparation of the patient intravenous infusion solution [see Dosage and Administration (2.5)].
- •
- For nasogastric tube administration, the reconstituted solution should be administered within 1 hour of reconstitution [see Dosage and Administration (2.6 )].
- •
- Discard any unused portion of the reconstituted solution.
2.5 Dilution and Preparation Instructions for the Intravenous Administration of the CRESEMBA for Injection Formulation
- •
- Based on the adult or pediatric dosage regimen [see Dosage and Administration (2.2 and 2.3)], remove the appropriate volume of the reconstituted solution (74.4 mg/mL of isavuconazonium sulfate) from the vial and add it to an infusion bag containing 250 mL of compatible diluent [see Dosage and Administration (2.7)]. A smaller volume infusion bag of compatible diluent may be used as long as the final concentration does not exceed approximately 1.5 mg isavuconazonium sulfate per mL.
- •
- The diluted solution may show visible translucent to white particulates of isavuconazole (which will be removed by in-line filtration).
- •
- Use gentle mixing or roll bag to minimize the formation of particulates. Avoid unnecessary vibration or vigorous shaking of the solution.
- •
- Apply in-line filter with a microporous membrane pore size of 0.2 to 1.2 micron and in-line filter reminder sticker to the infusion bag.
- •
- Do not use a pneumatic transport system.
- •
- The intravenous administration should be completed within 6 hours of dilution at room temperature. If this is not possible, immediately refrigerate (2°C to 8°C / 36°F to 46°F) the infusion solution after dilution and complete the infusion within 24 hours. Do not freeze the infusion solution.
2.6 Preparation Instructions for the Nasogastric Tube Administration of the CRESEMBA for Injection Formulation
CRESEMBA for injection can be administered through a nasogastric tube as follows:
- •
- Utilizing aseptic technique, reconstitute one vial of CRESEMBA for injection (equivalent to 200 mg isavuconazole) with 5 mL of water for injection, USP [see Dosage and Administration (2.4)].
- •
- Based on the adult or pediatric (6 years of age to less than 18 years of age) CRESEMBA for injection dosage regimen [see Dosage and Administration (2.2, 2.3)], withdraw the appropriate volume of the reconstituted solution (74.4 mg/mL of isavuconazonium sulfate) from the vial using an appropriate syringe and needle. Discard the needle and cap the syringe.
- •
- To administer, remove the cap from the syringe containing the reconstituted solution and connect the syringe to the nasogastric (NG) tube to deliver the dose. After administering the dose, administer three 5 mL rinses to the NG tube with water [see Clinical Pharmacology (12.3)].
- •
- Administer the reconstituted solution via the nasogastric tube within 1 hour of reconstitution. Discard any unused portion of the reconstituted solution.
- •
- Do not administer CRESEMBA capsules through a nasogastric tube.
Close2.7 Compatibility for the Injection Formulation
CRESEMBA for injection should only be administered with the following diluents:
- •
- 0.9% sodium chloride injection, USP
- •
- 5% dextrose injection, USP
-
3 DOSAGE FORMS AND STRENGTHSCRESEMBA Capsules - CRESEMBA 74.5 mg isavuconazonium sulfate (equivalent to 40 mg of isavuconazole) capsules are opaque and have a Swedish orange (reddish-brown) body imprinted with the Astellas ...
CRESEMBA Capsules
CRESEMBA 74.5 mg isavuconazonium sulfate (equivalent to 40 mg of isavuconazole) capsules are opaque and have a Swedish orange (reddish-brown) body imprinted with the Astellas logo in black ink and a Swedish orange cap imprinted with “557” in black ink.
CRESEMBA 186 mg isavuconazonium sulfate (equivalent to 100 mg of isavuconazole) capsules are opaque, elongated and have a Swedish orange (reddish-brown) body imprinted with the Astellas logo in black ink and a white cap imprinted with “766” in black ink.
CRESEMBA for Injection
Each single-dose vial of CRESEMBA for injection contains 372 mg isavuconazonium sulfate (equivalent to 200 mg of isavuconazole). CRESEMBA for injection is supplied in a single-dose vial as a sterile lyophilized white to yellow powder.
Close -
4 CONTRAINDICATIONS• CRESEMBA is contraindicated in persons with known hypersensitivity to isavuconazole. • Coadministration of strong CYP3A4 inhibitors, such as ketoconazole or high-dose ritonavir (400 mg every ...
- •
- CRESEMBA is contraindicated in persons with known hypersensitivity to isavuconazole.
- •
- Coadministration of strong CYP3A4 inhibitors, such as ketoconazole or high-dose ritonavir (400 mg every 12 hours), with CRESEMBA is contraindicated because strong CYP3A4 inhibitors can significantly increase the plasma concentration of isavuconazole [see Drug Interactions (7) and Clinical Pharmacology (12.3)].
- •
- Coadministration of strong CYP3A4 inducers, such as rifampin, carbamazepine, St. John’s wort, or long acting barbiturates with CRESEMBA is contraindicated because strong CYP3A4 inducers can significantly decrease the plasma concentration of isavuconazole [see Drug Interactions (7) and Clinical Pharmacology (12.3)].
- •
- CRESEMBA shortened the QTc interval in a concentration-related manner. CRESEMBA is contraindicated in patients with familial short QT syndrome [see Clinical Pharmacology (12.2)].
-
5 WARNINGS AND PRECAUTIONS5.1 Hepatic Adverse Drug Reactions - Hepatic adverse drug reactions (e.g., elevations in alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, total bilirubin ...
5.1 Hepatic Adverse Drug Reactions
Hepatic adverse drug reactions (e.g., elevations in alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, total bilirubin) have been reported in clinical trials. The elevations in liver-related laboratory tests were generally reversible and did not require discontinuation of CRESEMBA. Cases of more severe hepatic adverse drug reactions including hepatitis, cholestasis or hepatic failure including death have been reported in patients with serious underlying medical conditions (e.g., hematologic malignancy) during treatment with azole antifungal agents, including CRESEMBA.
Evaluate liver-related laboratory tests at the start and during the course of CRESEMBA therapy. Monitor patients who develop abnormal liver-related laboratory tests during CRESEMBA therapy for the development of more severe hepatic injury. Discontinue CRESEMBA if clinical signs and symptoms consistent with liver disease develop that may be attributable to CRESEMBA [see Adverse Reactions (6.1)].
5.2 Infusion-Related Reactions
Infusion-related reactions including hypotension, dyspnea, chills, dizziness, paresthesia, and hypoesthesia were reported during intravenous administration of CRESEMBA. Discontinue the infusion if these reactions occur [see Adverse Reactions (6.1)].
5.3 Hypersensitivity Reactions
Anaphylactic Reactions
Anaphylactic reactions, with fatal outcome, have been reported during treatment with CRESEMBA. Symptoms including dyspnea, hypotension, generalized erythema with flushing, and urticaria have been reported in such cases often soon after the initiation of treatment.
Severe Skin Reactions
Severe skin reactions, such as Stevens-Johnson syndrome, have been reported during treatment with other azole antifungal agents.
Discontinue CRESEMBA if a patient develops an anaphylactic or severe cutaneous adverse reaction and initiate supportive treatment as needed. There is no information regarding cross-sensitivity between CRESEMBA and other azole antifungal agents though cross-sensitivity between other triazole agents has been reported. When prescribing CRESEMBA to patients with hypersensitivity to other azoles, monitor for signs and symptoms of hypersensitivity reactions.
5.4 Embryo-Fetal Toxicity
Based on findings from animal reproduction studies, CRESEMBA may cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus.
Perinatal mortality was significantly increased in the offspring of pregnant rats dosed orally with isavuconazonium sulfate at 90 mg/kg/day (less than half the maintenance human dose based on AUC comparisons) during pregnancy through the weaning period.
Isavuconazonium chloride administration was associated with dose-related increases in the incidences of rudimentary cervical ribs in rats and rabbits at 30 and 45 mg/kg, respectively, doses equivalent to about 0.2 and 0.1 of the human maintenance dose based on AUC comparisons. In rats, dose-related increases in the incidences of zygomatic arch fusion and supernumerary ribs/rudimentary supernumerary ribs were also noted at 30 mg/kg and above, equivalent to one fifth the maintenance human dose based on AUC comparisons [see Use in Specific Populations (8.1)].
Advise females of reproductive potential to use an effective method of contraception during treatment with CRESEMBA and for 28 days after the final dose [see Use in Specific Populations (8.3)].
5.5 Drug Interactions
Coadministration of CRESEMBA with strong CYP3A4 inhibitors such as ketoconazole or high-dose ritonavir and strong CYP3A4 inducers such as rifampin, carbamazepine, St. John’s wort, or long acting barbiturates is contraindicated [see Contraindications (4) and Drug Interactions (7)].
Close5.6 Drug Particulates
Following dilution, CRESEMBA intravenous formulation may form precipitate from the insoluble isavuconazole. Administer CRESEMBA through an in-line filter [see Dosage and Administration (2.4)].
-
6 ADVERSE REACTIONSThe following are discussed in more detail in other sections of the labeling: • Hepatic Adverse Drug Reactions [see Warnings and Precautions (5.1)] • Infusion-Related Reactions [see Warnings and ...
The following are discussed in more detail in other sections of the labeling:
- •
- Hepatic Adverse Drug Reactions [see Warnings and Precautions (5.1)]
- •
- Infusion-Related Reactions [see Warnings and Precautions (5.2)]
- •
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
- •
- Embryo-Fetal Toxicity [see Warnings and Precautions (5.4)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of CRESEMBA cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trials Experience in Adult Patients
A total of 403 adult patients were exposed to CRESEMBA in two clinical trials. The most frequently reported adverse reactions among CRESEMBA-treated patients were nausea (26%), vomiting (25%), diarrhea (22%), headache (17%), elevated liver chemistry tests (16%), hypokalemia (14%), constipation (13%), dyspnea (12%), cough (12%), peripheral edema (11%), and back pain (10%). Serious adverse reactions occurred in 223/403 (55%) of patients and 56/403 (14%) of patients permanently discontinued treatment with CRESEMBA due to an adverse reaction in the two trials. The adverse reactions which most often led to permanent discontinuation of CRESEMBA therapy during the clinical trials were confusional state (0.7%), acute renal failure (0.7%), increased blood bilirubin (0.5%), convulsion (0.5%), dyspnea (0.5%), epilepsy (0.5%), respiratory failure (0.5%), and vomiting (0.5%).
Patients in the clinical trials were immunocompromised with underlying conditions including hematological malignancy, neutropenia post-chemotherapy, graft-versus-host disease, and hematopoietic stem cell transplant. The patient population was 61% male, had a mean age of 51 years (range 17-92, including 85 patients aged greater than 65 years), and was 79% white and 3% black. One hundred forty-four (144) patients had a duration of CRESEMBA therapy of greater than 12 weeks, with 52 patients receiving CRESEMBA for over six months.
In Trial 1, a randomized, double-blind, active-controlled clinical trial for treatment of invasive aspergillosis, treatment-emergent adverse reactions occurred in 247/257 (96%), and 255/259 (99%) patients in the CRESEMBA and voriconazole treatment groups, respectively. Adverse reactions resulting in permanent discontinuation were reported in 37 (14%) CRESEMBA-treated patients and 59 (23%) voriconazole-treated patients. Table 3 includes selected adverse reactions which were reported at an incidence of ≥ 5% during CRESEMBA therapy in Trial 1.
In Trial 2, an open-label, non-comparative trial of CRESEMBA in patients with invasive aspergillosis and renal impairment or invasive mucormycosis, adverse reactions occurred in 139/146 (95%) of patients in the CRESEMBA treatment group. Adverse reactions resulting in permanent discontinuation were reported in 19 (13%) CRESEMBA-treated patients. The frequencies and types of adverse reactions observed in CRESEMBA-treated patients were similar between Trial 1 and Trial 2.
Table 3. Selected Adverse Reactions with Rates of 5% or Greater in CRESEMBA-treated Patients in Trial 1 System Organ Class
Adverse ReactionsTrial 1 CRESEMBA
(N=257)
n (%)Voriconazole
(N=259)
n (%)- *
- Elevated liver laboratory tests include reactions of increased alanine aminotransferase, aspartate aminotransferase, blood alkaline phosphatase, blood bilirubin, and gamma-glutamyl transferase.
- †
- Delirium includes adverse reactions of agitation, confusional state, delirium, disorientation, and mental status changes.
Gastrointestinal disorders
Nausea
71 (27.6)
78 (30.1)
Vomiting
64 (24.9)
73 (28.2)
Diarrhea
61 (23.7)
60 (23.2)
Abdominal pain
43 (16.7)
59 (22.8)
Constipation
36 (14.0)
54 (20.8)
Dyspepsia
16 (6.2)
14 (5.4)
General disorders and administration site conditions
Edema peripheral
39 (15.2)
46 (17.8)
Fatigue
27 (10.5)
18 (6.9)
Chest pain
23 (8.9)
16 (6.2)
Injection site reaction
16 (6.2)
4 (1.5)
Hepatobiliary disorders
Elevated liver laboratory tests*
44 (17.1)
63 (24.3)
Metabolism and nutrition disorders
Hypokalemia
49 (19.1)
58 (22.4)
Decreased appetite
22 (8.6)
28 (10.8)
Hypomagnesemia
14 (5.4)
27 (10.4)
Musculoskeletal and connective tissue disorders
Back pain
26 (10.1)
19 (7.3)
Nervous system disorders
Headache
43 (16.7)
38 (14.7)
Psychiatric disorders
Insomnia
27 (10.5)
25 (9.7)
Delirium†
22 (8.6)
30 (11.6)
Anxiety
21 (8.2)
18 (6.9)
Renal and urinary disorders
Renal failure
26 (10.1)
21 (8.1)
Respiratory, thoracic and mediastinal disorders
Dyspnea
44 (17.1)
35 (13.5)
Acute respiratory failure
19 (7.4)
22 (8.5)
Skin and subcutaneous tissue disorders
Rash
22 (8.6)
36 (13.9)
Pruritus
21 (8.2)
15 (5.8)
Vascular disorders
Hypotension
21 (8.2)
28 (10.8)
The following adverse reactions occurred in less than 5% of all CRESEMBA-treated patients in Trial 1 or 2. The list does not include reactions presented in Table 3. This listing includes adverse reactions where a causal relationship to CRESEMBA cannot be ruled out or those which may help the physician in managing the risks to the patients.
- •
- Blood and lymphatic system disorders: agranulocytosis, leukopenia, pancytopenia
- •
- Cardiac disorders: atrial fibrillation, atrial flutter, bradycardia, reduced QT interval on electrocardiogram, palpitations, supraventricular extrasystoles, supraventricular tachycardia, ventricular extrasystoles, cardiac arrest
- •
- Ear and labyrinth disorders: tinnitus, vertigo
- •
- Eye disorders: optic neuropathy
- •
- Gastrointestinal disorders: abdominal distension, gastritis, gingivitis, stomatitis
- •
- General disorders and administration site conditions: catheter thrombosis, malaise, chills
- •
- Hepatobiliary disorders: cholecystitis, cholelithiasis, hepatitis, hepatomegaly, hepatic failure
- •
- Immune system disorders: hypersensitivity
- •
- Injury, poisoning and procedural complications: fall
- •
- Metabolism and nutrition disorders: hypoalbuminemia, hypoglycemia, hyponatremia
- •
- Musculoskeletal and connective tissue disorders: myositis, bone pain, neck pain
- •
- Nervous system disorders: convulsion, dysgeusia, encephalopathy, hypoesthesia, migraine, peripheral neuropathy, paresthesia, somnolence, stupor, syncope, tremor
- •
- Psychiatric disorders: confusion, hallucination, depression
- •
- Renal and urinary disorders: hematuria, proteinuria
- •
- Respiratory, thoracic and mediastinal disorders: bronchospasm, tachypnea
- •
- Skin and subcutaneous tissue disorders: alopecia, dermatitis, exfoliative dermatitis, erythema, petechiae, urticaria
- •
- Vascular disorders: thrombophlebitis
Laboratory Effects
In Trial 1, elevated liver transaminases (alanine aminotransferase or aspartate aminotransferase) greater than three times the upper limit of normal were reported at the end of study treatment in 4.4% of patients who received CRESEMBA. Elevations of liver transaminases greater than ten times the upper limit of normal developed in 1.2% of patients who received CRESEMBA.Clinical Trials Experience in Pediatric Patients
The clinical safety of CRESEMBA was assessed in 77 pediatric patients who received at least one dose of intravenous or oral CRESEMBA in two uncontrolled studies. Fifteen (19.5%) subjects were in the 1 to < 6 years old cohort, 30 subjects (39.0%) were in the 6 to < 12 years old cohort, and 32 subjects (41.6%) were in the 12 to < 18 years old cohort. The duration of treatment ranged from 1 to 181 days with a median duration of treatment of 15 days. The most frequently reported adverse reactions were diarrhea (26%), abdominal pain (23%), vomiting (21%), elevated liver chemistry tests (18%), rash (14%), nausea (13%), pruritus (13%) and headache (12%). In general, adverse reactions (including serious adverse reactions and adverse reactions leading to permanent discontinuation of CRESEMBA) were similar to those reported in adults.
Close6.2 Post-Marketing Experience
The following additional adverse reactions have been identified during post-approval use of CRESEMBA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate the frequency or establish a causal relationship to drug exposure.
Immune system disorders: anaphylactic reaction
-
7 DRUG INTERACTIONSIsavuconazole is a sensitive substrate of CYP3A4. CYP3A4 inhibitors or inducers may alter the plasma concentrations of isavuconazole. Isavuconazole is a moderate inhibitor of CYP3A4, and a mild ...
Isavuconazole is a sensitive substrate of CYP3A4. CYP3A4 inhibitors or inducers may alter the plasma concentrations of isavuconazole.
Isavuconazole is a moderate inhibitor of CYP3A4, and a mild inhibitor of P-glycoprotein (P-gp), and organic cation transporter 2 (OCT2).
Drug interaction studies were conducted to investigate the effect of coadministered drugs on the pharmacokinetics of isavuconazole and the effect of isavuconazole on the pharmacokinetics of coadministered drugs [see Clinical Pharmacology (12.3)].
Table 4. Drug(s) Affecting Pharmacokinetics of CRESEMBA Recommendation Comments - *
- 400 mg of lopinavir in combination with 100 mg of ritonavir.
Ketoconazole
Contraindicate coadministration of all potent CYP3A4 inhibitors
There is more than a 5-fold increase in exposure of isavuconazole upon coadministration with ketoconazole
[see Clinical Pharmacology (12.3)].Lopinavir/ritonavir*
Caution is advised when CRESEMBA is coadministered with lopinavir/ritonavir
There is a 96% increase in exposure of isavuconazole when coadministered with lopinavir/ritonavir
[see Clinical Pharmacology (12.3)].Rifampin
Contraindicate coadministration of all potent CYP3A4 inducers
There is a 97% decrease in exposure of isavuconazole upon coadministration with rifampin
[see Clinical Pharmacology (12.3)].CloseTable 5. The Effect of CRESEMBA on the Pharmacokinetics of Other Drugs Recommendation Comments - *
- 400 mg of lopinavir in combination with 100 mg of ritonavir.
Lopinavir/ritonavir*
Use with Caution
Concomitant administration of lopinavir/ritonavir and CRESEMBA resulted in decreased exposure of lopinavir and ritonavir that could possibly result in loss of antiviral efficacy [see Clinical Pharmacology (12.3)].
Atorvastatin
Use with Caution
Caution should be used when atorvastatin is used with CRESEMBA due to a potential increase in atorvastatin exposure. Monitor patients for adverse reactions that are typical of atorvastatin [see Clinical Pharmacology (12.3)].
Cyclosporine
Use with Caution
Concomitant administration of CRESEMBA and cyclosporine results in increase in cyclosporine exposure. Monitor drug concentrations of cyclosporine and adjust dose as needed
[see Clinical Pharmacology (12.3)].Sirolimus
Use with Caution
Concomitant administration of CRESEMBA and sirolimus results in increase in sirolimus exposure. Monitor drug concentrations of sirolimus and adjust dose as needed
[see Clinical Pharmacology (12.3)].Tacrolimus
Use with Caution
Concomitant administration of CRESEMBA and tacrolimus results in increase in tacrolimus exposure. Monitor drug concentrations of tacrolimus and adjust dose as needed
[see Clinical Pharmacology (12.3)].Midazolam
Use with Caution
Concomitant administration of CRESEMBA and midazolam results in increase in midazolam exposure. Consider dose reduction of midazolam when isavuconazole is coadministered [see Clinical Pharmacology (12.3)].
Bupropion
Use with Caution
Concomitant administration of CRESEMBA and bupropion results in decrease in bupropion exposure. Dose increase of bupropion may be necessary when coadministered with CRESEMBA, but should not exceed the maximum recommended dose [see Clinical Pharmacology (12.3)].
Mycophenolate Mofetil
Use with Caution
Concomitant administration of CRESEMBA and MMF results in increase in MMF exposure. Patients receiving CRESEMBA concurrently with MMF should be monitored for MPA-related toxicities [see Clinical Pharmacology (12.3)].
Digoxin
Use with Caution
Concomitant administration of CRESEMBA and digoxin results in increase in digoxin exposure. Serum digoxin concentrations should be monitored and used for titration when dosed concurrently with CRESEMBA [see Clinical Pharmacology (12.3)].
Vincristine
Avoid Concomitant Use
Avoid concomitant use with CRESEMBA in pediatric and adult patients. CRESEMBA is predicted to have a less than 2-fold increase in vincristine exposure in pediatric and adult patients [see Clinical Pharmacology (12.3)], which may increase the risk of vincristine-related adverse reactions.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings from animal studies, CRESEMBA may cause fetal harm when administered to a pregnant woman. There are no available human data on the use of CRESEMBA ...
8.1 Pregnancy
Risk Summary
Based on findings from animal studies, CRESEMBA may cause fetal harm when administered to a pregnant woman. There are no available human data on the use of CRESEMBA in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. In animal reproduction studies, perinatal mortality was increased in the offspring of pregnant rats dosed orally with isavuconazonium sulfate at approximately 0.5 times the clinical exposure during pregnancy through the weaning period. In animal studies when isavuconazonium chloride was administered by oral gavage to pregnant rats and rabbits during organogenesis at exposures corresponding to less than the human maintenance dose, increases in the incidences of multiple skeletal abnormalities, including rudimentary cervical ribs and fused zygomatic arches, were observed (see Data). Advise pregnant women of the potential risk to a fetus [see Warnings and Precautions (5.4)].
The estimated background risk of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Isavuconazonium chloride administration during organogenesis (gestational days 6-17 in rats and gestational days 6-18 in rabbits) was associated with dose-related increases in the incidences of rudimentary cervical ribs in rats and rabbits at 30 and 45 mg/kg, respectively, equivalent to about 0.2 and 0.1 times of the clinical exposure based on AUC comparisons. In rats, dose-related increases in the incidences of zygomatic arch fusion and supernumerary ribs/rudimentary supernumerary ribs were also noted at 30 mg/kg and above, equivalent to 0.2 times the human AUC. Skeletal abnormalities have also been observed in embryo-fetal development studies of other azole antifungal agents.
Isavuconazonium sulfate increased perinatal mortality in the pups when orally administered to pregnant rats during pregnancy and lactation (gestational day 6 through postpartum day 20) at doses up to 90 mg/kg/day (approximately 0.5 times the clinical exposure based on AUC comparison). No effect on the duration of pregnancy or delivery was seen in the pups at this same dose.
8.2 Lactation
Risk Summary
There are no data on the presence of isavuconazole in human milk, the effects on the breastfed infant or the effects on milk production. Isavuconazole was present in the milk of lactating rats following intravenous administration. When a drug is present in animal milk, it is likely that the drug will be present in human milk. Therefore, breastfeeding should be discontinued during treatment with CRESEMBA.
8.3 Females and Males of Reproductive Potential
Contraception
CRESEMBA may cause embryo-fetal harm when administered to pregnant women [see Warnings and Precautions (5.4) and Use in Specific Populations (8.1)]. Advise female patients of reproductive potential to use effective contraception during treatment with CRESEMBA and for 28 days after the final dose.
8.4 Pediatric Use
Invasive Aspergillosis
The safety and effectiveness of CRESEMBA for injection for the treatment of invasive aspergillosis have been established in pediatric patients 1 year of age and older. The safety and effectiveness of CRESEMBA capsules for the treatment of invasive aspergillosis have been established in pediatric patients 6 year of age and older weighing 16 kg and greater. Use of CRESEMBA in this age group for treatment of invasive aspergillosis is supported by evidence from one adequate and well-controlled trial in adult patients and additional pharmacokinetic and safety data in pediatric patients 1 year of age and older [see Clinical Pharmacology (12.3)]. Adverse reactions in this pediatric population were similar to those reported in the adult population [see Adverse Reactions (6.1)]. The safety and effectiveness of CRESEMBA capsules for treatment of invasive aspergillosis have not been established in pediatric patients younger than 6 years of age or who weigh less than 16 kg because the oral route of administration was not assessed in this pediatric patient age cohort.
The safety and effectiveness of CRESEMBA for treatment of invasive aspergillosis in pediatric patients less than 1 year of age have not been established.
Invasive Mucormycosis
The safety and effectiveness of CRESEMBA for injection for the treatment of invasive mucormycosis have been established in pediatric patients 1 year of age and older. The safety and effectiveness of CRESEMBA capsules for the treatment of invasive mucormycosis have been established in pediatric patients 6 year of age and older weighing 16 kg and greater. Use of CRESEMBA in this age group for treatment of invasive mucormycosis is supported by one open-label trial in adult patients with invasive mucormycosis, a retrospective review of survival data for adult patients with untreated invasive mucormycosis, and additional pharmacokinetic and safety data in pediatric patients 1 year of age and older [see Clinical Pharmacology (12.3)].Adverse reactions in this pediatric population were similar to those reported in the adult population [see Adverse Reactions (6.1)]. The safety and effectiveness of CRESEMBA capsules for treatment of invasive mucormycosis have not been established in pediatric patients younger than 6 years of age who weigh less than 16 kg because the oral route of administration was not assessed in this pediatric patient age cohort.
The safety and effectiveness of CRESEMBA for treatment of invasive mucormycosis in pediatric patients less than 1 year of age have not been established.
8.5 Geriatric Use
Of the 547 patients who received CRESEMBA in the Phase 2 and 3 trials, 86 (16%) of patients were greater than 65 years of age and 20 (4%) were greater than 75 years of age. The pharmacokinetics of isavuconazole are comparable in young and elderly subjects (65 years of age and older) [see Clinical Pharmacology (12.3)]. No dose adjustment of CRESEMBA is needed in elderly patients.
No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
Of the 403 patients who received CRESEMBA in the Phase 3 trials, 79 (20%) of patients had an estimated glomerular filtration rate (GFR) less than 60 mL/min/1.73 m2. No dose adjustment is needed in patients with mild, moderate, or severe renal impairment, including those patients with End-Stage Renal Disease (ESRD) [see Clinical Pharmacology (12.3)].
Close8.7 Hepatic Impairment
No dose adjustment is necessary in patients with mild or moderate hepatic impairment (Child-Pugh Class A and B) [see Clinical Pharmacology (12.3)]. CRESEMBA has not been studied in patients with severe hepatic impairment (Child-Pugh Class C) and should be used in these patients only when the benefits outweigh the risks. Clinical monitoring for CRESEMBA-related adverse reactions is recommended when treating patients with severe hepatic impairment [see Warnings and Precautions (5.1)].
-
10 OVERDOSAGEDuring clinical studies, total daily CRESEMBA doses higher than the recommended dose regimen were associated with an increased rate of adverse reactions. At supratherapeutic doses (three times the ...
During clinical studies, total daily CRESEMBA doses higher than the recommended dose regimen were associated with an increased rate of adverse reactions. At supratherapeutic doses (three times the recommended maintenance dose) evaluated in a thorough QT study, there were proportionally more treatment-emergent adverse reactions than in the therapeutic dose group (maintenance dose) for the following: headache, dizziness, paresthesia, somnolence, disturbance in attention, dysgeusia, dry mouth, diarrhea, oral hypoesthesia, vomiting, hot flush, anxiety, restlessness, palpitations, tachycardia, photophobia and arthralgia. Adverse reactions leading to discontinuation of study drug occurred in 7 of 39 (17.9%) subjects in the supratherapeutic dose group.
Isavuconazole is not removed by hemodialysis. There is no specific antidote for isavuconazole. Treatment should be supportive with appropriate monitoring.
Close -
11 DESCRIPTIONCRESEMBA contains isavuconazonium sulfate, which is the prodrug of isavuconazole, an azole antifungal drug. Isavuconazonium sulfate drug substance is an amorphous, white to yellowish-white powder ...
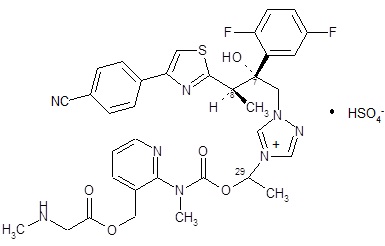
CRESEMBA contains isavuconazonium sulfate, which is the prodrug of isavuconazole, an azole antifungal drug. Isavuconazonium sulfate drug substance is an amorphous, white to yellowish-white powder. The chemical name of isavuconazonium sulfate is glycine, N-methyl-, [2-[[[1-[1-[(2R,3R)-3-[4-(4-cyanophenyl)-2-thiazolyl]-2-(2,5-difluorophenyl)-2-hydroxybutyl]-4H-1,2,4-triazolium-4-yl]ethoxy]carbonyl]methylamino]-3-pyridinyl]methyl ester, sulfate (1:1). The empirical formula is C35H35F2N8O5S·HSO4, the molecular weight is 814.84 and the structural formula is:
CRESEMBA Capsules
CRESEMBA (isavuconazonium sulfate) 74.5 mg capsules are available for oral administration. Each CRESEMBA capsule contains 74.5 mg isavuconazonium sulfate, equivalent to 40 mg isavuconazole. The inactive ingredients include black iron oxide, colloidal silicon dioxide, disodium edetate, gellan gum, hypromellose, magnesium citrate, microcrystalline cellulose, potassium acetate, potassium hydroxide, propylene glycol, purified water, red iron oxide, shellac, sodium lauryl sulfate, stearic acid, strong ammonia solution, talc and titanium dioxide.
CRESEMBA (isavuconazonium sulfate) 186 mg capsules are available for oral administration. Each CRESEMBA capsule contains 186 mg isavuconazonium sulfate, equivalent to 100 mg isavuconazole. The inactive ingredients include black iron oxide, colloidal silicon dioxide, disodium edetate, gellan gum, hypromellose, magnesium citrate, microcrystalline cellulose, potassium acetate, potassium hydroxide, propylene glycol, purified water, red iron oxide, shellac, sodium lauryl sulfate, stearic acid, strong ammonia solution, talc and titanium dioxide.
CRESEMBA for Injection
CRESEMBA (isavuconazonium sulfate) for injection is available for intravenous administration. CRESEMBA for injection is a white to yellow sterile, lyophilized powder containing 372 mg isavuconazonium sulfate, equivalent to 200 mg isavuconazole, per vial. Inactive ingredients included in each vial are 96 mg mannitol and sulfuric acid for pH adjustment.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Isavuconazonium sulfate is the prodrug of isavuconazole, an azole antifungal [see Microbiology (12.4)]. 12.2 Pharmacodynamics - Pharmacokinetic/Pharmacodynamic ...
12.1 Mechanism of Action
Isavuconazonium sulfate is the prodrug of isavuconazole, an azole antifungal [see Microbiology (12.4)].
12.2 Pharmacodynamics
Pharmacokinetic/Pharmacodynamic Relationship
In patients treated with CRESEMBA for invasive aspergillosis in a controlled trial, there was no significant association between plasma AUC or plasma isavuconazole concentration and efficacy.
Cardiac Electrophysiology
The effect on QTc interval of multiple doses of CRESEMBA capsules was evaluated. CRESEMBA was administered as 2 capsules (equivalent to 200 mg isavuconazole) three times daily on days 1 and 2 followed by either 2 capsules or 6 capsules (equivalent to 600 mg isavuconazole) once daily for 13 days in a randomized, placebo- and active-controlled (moxifloxacin 400 mg single-dose), four-treatment-arm, parallel study in 160 healthy subjects.
Isavuconazole resulted in dose-related shortening of the QTc interval. For the 2-capsule dosing regimen, the least squares mean (LSM) difference from placebo was -13.1 msec at 2 hours postdose [90% CI: -17.1, -9.1 msec]. Increasing the dose to 6 capsules resulted in an LSM difference from placebo of -24.6 msec at 2 hours postdose [90% CI: -28.7, -20.4]. CRESEMBA was not evaluated in combination with other drugs that reduce the QTc interval, so the additive effects are not known.
12.3 Pharmacokinetics
General Pharmacokinetics
In healthy subjects, the pharmacokinetics of isavuconazole following oral administration of CRESEMBA capsules at isavuconazole equivalent doses up to 600 mg per day (6 capsules) are dose proportional (Table 6). Based on a population pharmacokinetics analysis of healthy subjects and patients, the mean plasma half-life of isavuconazole was 130 hours and the mean volume of distribution (Vss) was approximately 450 L following intravenous administration.
Table 6. Steady State Pharmacokinetic Parameters of Isavuconazole Following Administration of 186 mg CRESEMBA Capsules - *
- Each capsule contains the equivalent of 100 mg of isavuconazole.
Parameter
CRESEMBA 186 mg
2 Capsules*(n = 37)
CRESEMBA 186 mg
6 Capsules*(n = 32)
Cmax (mg/L)
Mean
SD
CV %
7.5
1.9
25.2
20.0
3.6
17.9
tmax (hr)
Median
Range
3.0
2.0 – 4.0
4.0
2.0 – 4.0
AUC (mg•hr/L)
Mean
SD
CV %
121.4
35.8
29.5
352.8
72.0
20.4
Following oral administration of CRESEMBA capsules at an isavuconazole equivalent dose of 200 mg in 66 fasted healthy male subjects, a single dose administration of two 186 mg CRESEMBA capsules and five 74.5 mg CRESEMBA capsules exhibited a mean (SD) Cmax and AUC of 3.3 (0.6) mg/L and 112.2 (30.3) mg·hr/L, respectively, and 3.3 (0.6) mg/L and 118.0 (33.1) mg·hr/L, respectively.
Absorption
After oral administration of CRESEMBA in healthy volunteers, the active moiety, isavuconazole, generally reaches maximum plasma concentrations (Cmax) 2 hours to 3 hours after single and multiple dosing. The absolute bioavailability of isavuconazole following oral administration of CRESEMBA is 98%. No significant concentrations of the prodrug or inactive cleavage product were seen in plasma after oral administration.
Following intravenous administration of CRESEMBA, maximal plasma concentrations of the prodrug and inactive cleavage product were detectable during infusion and declined rapidly following the end of administration. The prodrug was below the level of detection by 1.25 hours after the start of a one-hour infusion. The total exposure of the prodrug based on AUC was less than 1% that of isavuconazole. The inactive cleavage product was quantifiable in some subjects up to 8 hours after the start of infusion. The total exposure of inactive cleavage product based on AUC was approximately 1.3% that of isavuconazole.
CRESEMBA given orally as an intravenous solution administered via nasogastric (NG) tube provides systemic isavuconazole exposure that is similar to the oral capsule (Table 7).
Table 7. Statistical Comparison of Plasma Pharmacokinetics of Isavuconazole Following Single Oral Dose Administration of 2 Capsules of 186 mg (Equivalent to 200 mg Isavuconazole) and Single Intravenous Solution Dose Administration of 372 mg (Equivalent to 200 mg Isavuconazole) via Nasogastric (NG) Tube in Healthy Adult Subjects Under Fasted Conditions CRESEMBA IV Solution via NG Tube
CRESEMBA Oral Capsules
NG Tube/Oral Capsule
Pharmacokinetic Parameter
N
Mean (%CV)
N
Mean (%CV)
GMR (90% CI)
Cmax (mg/L)
13
2.3 (23.6)
13
2.2 (26.7)
105.34 (89-124)
AUC0-72hr
(mg·hr/L)
13
34.9 (22.1)
13
35.8 (24.6)
97.81 (93-103)
AUC0-∞
(mg·hr/L)
12
98.1 (44.5)
12
100.1 (46.8)
99.27 (93-106)
GMR = Geometric least-squares mean ratio; CI = confidence interval
Distribution
Isavuconazole is extensively distributed with a mean steady state volume of distribution (Vss) of approximately 450 L. Isavuconazole is highly protein bound (greater than 99%), predominantly to albumin.
Elimination
Metabolism
In in vitro studies isavuconazonium sulfate is rapidly hydrolyzed in blood to isavuconazole by esterases, predominantly by butylcholinesterase. Isavuconazole is a substrate of cytochrome P450 enzymes 3A4 and 3A5.
Following single doses of [cyano 14C] isavuconazonium and [pyridinylmethyl 14C] isavuconazonium in humans, in addition to the active moiety (isavuconazole) and the inactive cleavage product, a number of minor metabolites were identified. Except for the active moiety isavuconazole, no individual metabolite was observed with an AUC greater than 10% of drug-related material.
In vivo studies indicate that CYP3A4, CYP3A5 and subsequently uridine diphosphate-glucuronosyltransferases (UGT) are involved in the metabolism of isavuconazole.
Excretion
Following oral administration of radio-labeled isavuconazonium sulfate to healthy volunteers, a mean of 46.1% of the total radioactive dose was recovered in the feces and 45.5% was recovered in the urine.
Renal excretion of isavuconazole itself was less than 1% of the dose administered.
The inactive cleavage product is primarily eliminated by metabolism and subsequent renal excretion of the metabolites. Renal elimination of intact cleavage product was less than 1% of the total dose administered. Following intravenous administration of radio-labeled cleavage product, 95% of the total radioactive dose was excreted in the urine.
Special populations
Geriatric Patients
The AUC of isavuconazole following a single oral dose of CRESEMBA equivalent to 200 mg isavuconazole in elderly subjects (65 years and older) was similar to that in younger volunteers (18 years to 45 years). The AUC was similar between younger female and male subjects and between elderly and younger males.
Elderly female AUC estimates were 38% and 47% greater than AUC estimates obtained in elderly males and younger females, respectively. The pharmacokinetic difference in elderly females receiving CRESEMBA are not considered to be clinically significant. Therefore, no dose adjustment is required based on age and gender.
Pediatric Patients
The pharmacokinetics of isavuconazole were evaluated in two clinical studies (N = 73) in pediatric patients aged 1 to less than 18 years of age which included twenty-eight patients with at least possible invasive aspergillosis or possible invasive mucormycosis.
Table 8. Derived Steady State Isavuconazole AUC (mg•hr/L) values in Pediatric Patients, by Age Group - *
- Estimated AUCss values of 15 mg/kg that were derived from existing values of pediatric patients that received 10 mg/kg of CRESEMBA for injection administered intravenously.
- †
- CRESEMBA for injection administered intravenously.
- ‡
- CRESEMBA for injection administered intravenously or CRESEMBA capsules administered orally.
Dosage
15 mg/kg*
10 mg/kg†
10 mg/kg
or
Maximum Dose of 372 mg‡
Age Group
1 to < 3 years
(n=5)
3 to < 6 years
(n=10)
6 to < 12 years
(n=29)
12 to < 18 years
(n=29)
Mean
80.2
103.3
97.3
104.2
Median
64.3
110.3
87.7
97.7
Minimum - Maximum
53.7 - 155
51.5 – 159.1
37.8– 153.8
35.5 – 215.6
Race
A 2-compartment population pharmacokinetic model was developed to assess the pharmacokinetics of isavuconazole between healthy Western and Chinese subjects. Chinese subjects were found to have on average a 40% lower clearance compared to Western subjects (1.6 L/hr for Chinese subjects as compared to 2.6 L/hr for Western subjects) and therefore approximately 50% higher AUC than Western subjects. Body mass index (BMI) did not play a role in the observed differences. No dose adjustment is recommended for Chinese patients.
Gender
AUC estimates were similar between young female and male subjects (18 years to 45 years). There was a difference in AUC for elderly females (see Geriatric section above). No dose adjustment is required based on gender.
Patients with Renal Impairment
Total isavuconazole AUC and Cmax were not affected to a clinically meaningful extent in subjects with mild, moderate and severe renal impairment relative to healthy controls. No dose adjustment is necessary in patients with renal impairment.
Isavuconazole is not readily dialyzable. A dose adjustment is not warranted in patients with ESRD.
Patients with Hepatic Impairment
After a single-dose of CRESEMBA equivalent to 100 mg of isavuconazole was administered to 32 patients with mild (Child-Pugh Class A) hepatic impairment and 32 patients with moderate (Child-Pugh Class B) hepatic impairment (16 intravenous and 16 oral patients per Child-Pugh Class), the least squares mean systemic exposure (AUC) increased 64% and 84% in the Child-Pugh Class A group and the Child-Pugh Class B group, respectively, relative to 32 age and weight-matched healthy subjects with normal hepatic function. Mean Cmax was 2% lower in the Child-Pugh Class A group and 30% lower in the Child-Pugh Class B group. The population pharmacokinetic evaluation of isavuconazole in healthy subjects and patients with mild and moderate hepatic impairment demonstrated that the mild and moderate hepatic impairment population had 40% and 48% lower isavuconazole clearance (CL) values, respectively, compared to the healthy population. It is recommended that the standard CRESEMBA loading dose and maintenance dose regimen be utilized in patients with mild to moderate hepatic disease. CRESEMBA has not been studied in patients with severe hepatic impairment (Child-Pugh Class C).
Drug Interaction Studies
In Vitro Studies
CYP450 Enzymes: Isavuconazole is a substrate of CYP3A4 and CYP3A5. Isavuconazole is an inhibitor of CYP3A4, CYP2C8, CYP2C9, CYP2C19, and CYP2D6. Isavuconazole is an inducer of CYP3A4, CYP2B6, CYP2C8, and CYP2C9.
Transporter Systems: Isavuconazole is an inhibitor of P-gp-, BCRP- and OCT2.
Clinical Studies and Model-Informed Approaches
The effect of coadministration of drugs on the pharmacokinetics of isavuconazole and the effect of isavuconazole on the pharmacokinetics of coadministered drugs were studied after single and multiple doses of isavuconazole in healthy subjects.
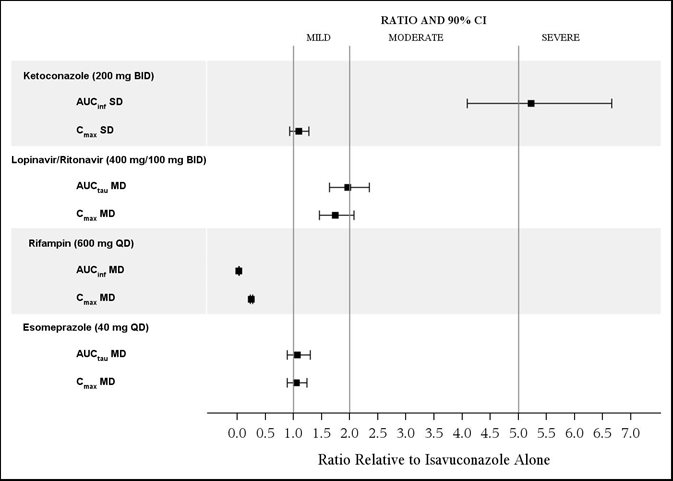
The effects of ketoconazole, rifampin, lopinavir/ritonavir, and esomeprazole on isavuconazole are shown in Figure 1.
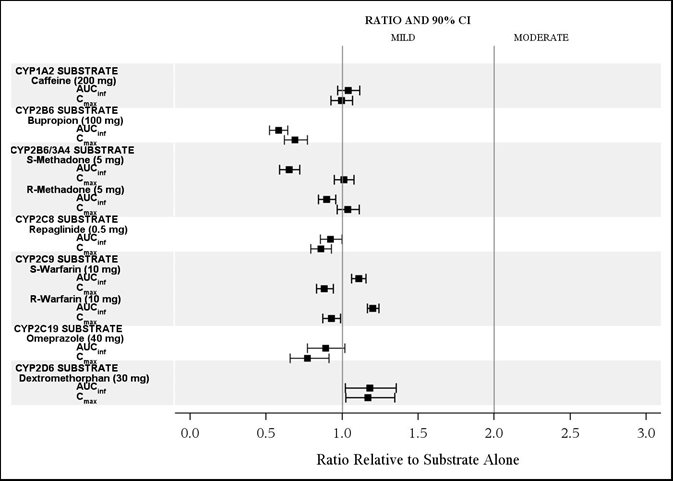
Ketoconazole: As a strong CYP3A4 inhibitor, ketoconazole increased the isavuconazole Cmax by 9% and isavuconazole AUC by 422% after multiple-dose administration of ketoconazole (200 mg twice daily) for 24 days and a single-dose of CRESEMBA equivalent to 200 mg of isavuconazole. Isavuconazole is a sensitive CYP3A4 substrate and use with strong CYP3A4 inhibitors are contraindicated per Section 4 and Figure 1.
Lopinavir/Ritonavir: Lopinavir/ritonavir (400 mg/100 mg twice daily) increased the Cmax and AUC of isavuconazole by 74% and 96%, respectively, with concurrent decreases in the mean AUCs of lopinavir and ritonavir by 27% and 31%, respectively.
Rifampin: Rifampin (600 mg) decreased the mean Cmax and AUC of isavuconazole by 75% and 97%, respectively, when coadministered with multiple doses of CRESEMBA and thus, coadministration of CRESEMBA with strong CYP3A4 inducers is contraindicated.
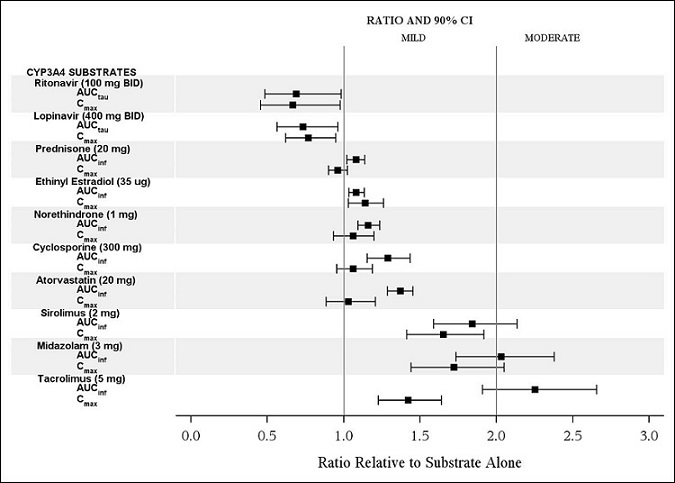
The effects of isavuconazole on ritonavir, lopinavir, prednisone, combined oral contraceptives (ethinyl estradiol and norethindrone), cyclosporine, atorvastatin, sirolimus, midazolam, and tacrolimus are shown in Figure 2.
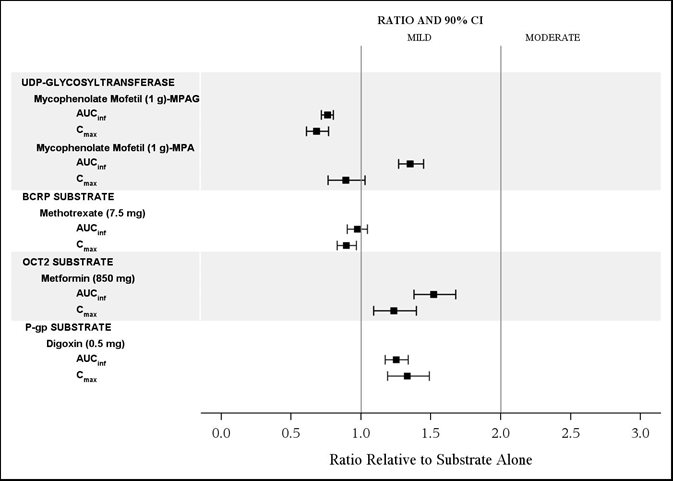
CYP3A4 Substrates: CRESEMBA increased the systemic exposure of sensitive CYP3A4 substrates midazolam, sirolimus and tacrolimus approximately 2-fold, and therefore CRESEMBA is a moderate inhibitor of CYP3A4.
The effects of isavuconazole on other CYP substrates: caffeine, bupropion, methadone, repaglinide, warfarin, omeprazole, and dextromethorphan, are shown in Figure 3.
The effects of isavuconazole on the substrates of UGT and transporters: mycophenolate mofetil (MMF), methotrexate, metformin, and digoxin are shown in Figure 4.
Vincristine: Vincristine (P-gp substrate) exposure is predicted to increase by less than 2-fold in pediatric and adult patients following concomitant administration with CRESEMBA.
Close12.4 Microbiology
Mechanism of Action
Isavuconazonium sulfate is the prodrug of isavuconazole, an azole antifungal drug. Isavuconazole inhibits the synthesis of ergosterol, a key component of the fungal cell membrane, through the inhibition of cytochrome P-450 dependent enzyme lanosterol 14-alpha-demethylase. This enzyme is responsible for the conversion of lanosterol to ergosterol. An accumulation of methylated sterol precursors and a depletion of ergosterol within the fungal cell membrane weakens the membrane structure and function. Mammalian cell demethylation is less sensitive to isavuconazole inhibition.
Resistance
There is a potential for development of resistance to isavuconazole.
The mechanism of resistance to isavuconazole, like other azole antifungals, is likely due to multiple mechanisms that include substitutions in the target gene CYP51. Changes in sterol profile and elevated efflux pump activity were observed; however, the clinical relevance of these findings is unclear.
In vitro and animal studies suggest cross-resistance between isavuconazole and other azoles. The relevance of cross-resistance to clinical outcome has not been fully characterized; however, patients failing prior azole therapy may require alternative antifungal therapy.
Antimicrobial Activity
Isavuconazole has activity against most strains of the following microorganisms, both in vitro and in clinical infections: Aspergillus flavus, Aspergillus fumigatus, Aspergillus niger, and Mucorales such as Rhizopus oryzae and Mucormycetes species [see Clinical Studies (14)].
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - In a 2-year rat carcinogenicity study and a 2-year mouse carcinogenicity study, dose-related increases in ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a 2-year rat carcinogenicity study and a 2-year mouse carcinogenicity study, dose-related increases in hepatocellular adenomas and/or carcinomas were observed in male and female B6C3F1/Crl mice, and male, but not female Han Wistar rats at doses as low as 0.1 times the exposure seen in humans administered the maintenance dose. Hepatic hemangiomas were increased in female mice at 300 mg/kg, at an exposure similar to the maintenance dose. Hepatoblastoma were increased in male mice at 100 mg/kg, about 0.4 times the systemic exposures based on AUC comparisons.
Thyroid follicular cell adenomas were observed in male and female rats at doses as low as 60 mg/kg in male rats (about 0.2 times the human clinical maintenance dose). The relevance of the rat thyroid tumors to human carcinogenic risk remains unclear.
A significant increase in the incidence of skin fibromas was seen in male rats at 300 mg/kg, exposures 0.8 times the human exposure at the human clinical maintenance dose. Uterine adenocarcinomas were observed in female rats at 200 mg/kg, at systemic exposures similar to the human exposure at the human clinical maintenance dose.
Mutagenesis
No mutagenic or clastogenic effects were detected in the in vitro bacterial reverse mutation assay and the in vivo bone marrow micronucleus assay in rats.
Impairment of Fertility
Oral administration of isavuconazonium sulfate did not affect the fertility in male or female rats treated at doses up to 90 mg/kg/day (approximately 0.3 times the systemic exposure at the human clinical maintenance dose).
-
14 CLINICAL STUDIES14.1 Treatment of Invasive Aspergillosis - Trial 1 was a randomized, double-blind, non-inferiority active controlled trial which evaluated the safety and efficacy of CRESEMBA versus voriconazole ...
14.1 Treatment of Invasive Aspergillosis
Trial 1 was a randomized, double-blind, non-inferiority active controlled trial which evaluated the safety and efficacy of CRESEMBA versus voriconazole for primary treatment of invasive fungal disease caused by Aspergillus species or other filamentous fungi. Eligible patients had proven, probable, or possible invasive fungal infections per European Organisation for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) criteria1. Patients were stratified by history of allogeneic bone marrow transplant, uncontrolled malignancy at baseline, and by geographic region. The mean age of patients was 51 years (range 17-87) and the majority were Caucasians (78%), male (60%), with fungal disease involving the lungs (95%). At least one Aspergillus species was identified in 30% of the subjects; A. fumigatus and A. flavus were the most common pathogens identified. There were few patients with other Aspergillus species:
A. niger, A. sydowii, A. terreus, and A. westerdijkiae. Baseline risk factors are presented in Table 9.Table 9. Baseline Risk Factors in Intent-To-Treat (ITT*) Population CRESEMBA
N=258
n (%)Voriconazole
N=258
n (%)Hematologic Malignancy
211 (82)
222 (86)
Allogeneic Hematopoietic Stem Cell Transplant
54 (21)
51 (20)
Neutropenia†
163 (63)
175 (68)
Corticosteroid Use
48 (19)
39 (15)
T-Cell Immunosuppressant Use
111 (43)
109 (42)
Patients randomized to receive CRESEMBA treatment were administered a loading dose intravenously of 372 mg of isavuconazonium sulfate (equivalent to 200 mg of isavuconazole) every 8 hours for the first 48 hours. Beginning on Day 3, patients received intravenous or oral therapy of 372 mg of isavuconazonium sulfate (equivalent to 200 mg of isavuconazole) once daily. Patients randomized to receive voriconazole treatment were administered voriconazole intravenously with a loading dose of 6 mg/kg every 12 hours for the first 24 hours followed by 4 mg/kg intravenously every 12 hours for the following 24 hours. Therapy could then be switched to an oral formulation of voriconazole at a dose of 200 mg every 12 hours. In this trial, the protocol-defined maximum treatment duration was 84 days. Mean treatment duration was 47 days for both treatment groups, of which 8 to 9 days was by an intravenous route of administration.
All-cause mortality through Day 42 in the overall population (ITT) was 18.6% in the CRESEMBA treatment group and 20.2% in the voriconazole treatment group for an adjusted treatment difference of -1.0% with 95% confidence interval of -8.0% to 5.9%. Similar results were seen in patients with proven or probable invasive aspergillosis confirmed by serology, culture or histology (see Table 10).
Table 10. All-Cause Mortality Through Day 42 - *
- Adjusted treatment difference (CRESEMBA-voriconazole) by Cochran-Mantel-Haenszel method stratified by the randomization factors.
CRESEMBA
Voriconazole
N
All-cause Mortality
n (%)N
All-cause Mortality
n (%)Difference*
(95% CI)%ITT
258
48 (18.6)
258
52 (20.2)
-1.0 (-8.0, 5.9)
Proven or Probable
Invasive Aspergillosis123
23 (18.7)
108
24 (22.2)
-2.7 (-13.6, 8.2)
Overall success at End-of-Treatment (EOT) was assessed by a blinded, independent Data Review Committee (DRC) using pre-specified clinical, mycological, and radiological criteria. In the subgroup of patients with proven or probable invasive aspergillosis confirmed by serology, culture or histology, overall success at EOT was seen in 35% of CRESEMBA-treated patients compared to 38.9% of voriconazole-treated patients (see Table 11).
Table 11. Overall Response Success at End-of-Treatment - *
- Adjusted treatment difference (CRESEMBA-voriconazole) by Cochran-Mantel-Haenszel method stratified by the randomization factors.
CRESEMBA
Voriconazole
N
Success
n (%)N
Success
n (%)Difference*
(95% CI)%Proven or Probable
Invasive Aspergillosis123
43 (35.0)
108
42 (38.9)
-4.0 (-16.3, 8.4)
Close14.2 Treatment of Invasive Mucormycosis
Trial 2, an open-label, non-comparative trial, evaluated the safety and efficacy of a subset of patients with invasive mucormycosis. Thirty-seven (37) patients had proven or probable mucormycosis according to criteria based on those established by the European Organisation for Research and Treatment of Cancer/Mycoses Study Group1. Rhizopus oryzae and Mucormycetes were the most common pathogens identified. There were few patients with other Mucorales: Lichtheimia corymbifera, Mucor amphibiorum, Mucor circinelloides, Rhizomucor pusillus, Rhizopus azygosporus, and Rhizopus microsporus. The patients were white (68%), male (81%), and had a mean age of 49 years (range 22-79). Fifty-nine percent (59%) of patients had pulmonary disease involvement, half of whom also had other organ involvement. The most common non-pulmonary disease locations were sinus (43%), eye (19%), CNS (16%) and bone (14%). Baseline risk factors are presented in Table 12. The Independent Data Review Committee classified patients receiving CRESEMBA as primary therapy, or for invasive mold disease refractory to, or patients intolerant of other antifungal therapy.
Table 12. Baseline Risk Factors in Mucorales Patients Therapy status assessed by the Independent Data Review Committee: Primary = patients received CRESEMBA as primary treatment; refractory = patient’s underlying infection not adequately treated by prior therapy; intolerant = patients unable to tolerate prior therapy. - *
- Neutropenia is defined as less than 500 cells/mm3.
Primary
N=21
n (%)Refractory
N=11
n (%)Intolerant
N=5
n (%)Total
N=37
n (%)Hematologic Malignancy
11 (52)
7 (64)
4 (80)
22 (60)
Allogeneic Hematopoietic Stem Cell Transplant
4 (19)
4 (36)
5 (100)
13 (35)
Neutropenia*
4 (19)
5 (46)
1 (20)
10 (27)
Corticosteroid Use
5 (24)
3 (27)
2 (40)
10 (27)
T-Cell Immunosuppressant Use
7 (33)
6 (55)
5 (100)
18 (49)
Diabetic
4 (19)
0
0
4 (11)
Patients were treated with CRESEMBA intravenously or via oral administration at the recommended doses. Median treatment duration was 102 days for patients classified as primary, 33 days for refractory, and 85 days for intolerant [see Dosage and Administration (2.2)].
For patients with invasive mucormycosis, all-cause mortality through Day 42 and success in overall response at the End-of-Treatment as assessed by the Independent Data Review Committee is shown in Table 13. These results provide evidence that CRESEMBA is effective for the treatment of mucormycosis, in light of the natural history of untreated mucormycosis. However, the efficacy of CRESEMBA for the treatment of invasive mucormycosis has not been evaluated in concurrent, controlled clinical trials.
Table 13. All-Cause Mortality and Overall Response Success in Mucorales Patients - *
- Two primary mucormycosis patients were not assessed at End-of-Treatment due to ongoing treatment.
Primary
N=21Refractory
N=11Intolerant
N=5Total
N=37All-cause Mortality
Through Day 427 (33%)
5 (46%)
2 (40%)
14 (38%)
Overall Response
Success Rate at
End-of-Treatment6/19* (32%)
4/11 (36%)
1/5 (20%)
11/35* (31%)
-
15 REFERENCES 1. DePauw, B., Walsh, T.J., Donnelly, J.P., et al. (2008) Revised Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer Invasive Fungal ...
- 1.
- DePauw, B., Walsh, T.J., Donnelly, J.P., et al. (2008) Revised Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer Invasive Fungal Infections Quadrature Group and National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) consensus group. Clinical Infectious Diseases 46:1813-1821.
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - CRESEMBA Capsules - CRESEMBA (isavuconazonium sulfate) 74.5 mg capsules are supplied as opaque capsules and have a Swedish orange (reddish-brown) body imprinted with the ...
16.1 How Supplied
CRESEMBA Capsules
CRESEMBA (isavuconazonium sulfate) 74.5 mg capsules are supplied as opaque capsules and have a Swedish orange (reddish-brown) body imprinted with the Astellas logo in black ink and a Swedish orange cap imprinted with “557” in black ink. Each capsule contains 74.5 mg isavuconazonium sulfate (equivalent to 40 mg of isavuconazole) and is packaged as follows:35-count carton (contains seven individual aluminum child-resistant blister packs of 5 capsules per sheet with desiccant)
NDC 0469-2860-35
CRESEMBA (isavuconazonium sulfate) 186 mg capsules are supplied as opaque and elongated capsules and have a Swedish orange (reddish-brown) body imprinted with the Astellas logo in black ink and a white cap imprinted with “766” in black ink. Each capsule contains 186 mg isavuconazonium sulfate (equivalent to 100 mg of isavuconazole) and is packaged as follows:
14-count carton (contains two individual aluminum child-resistant blister packs of 7 capsules per sheet with desiccant)
NDC 0469-0520-02
CRESEMBA for Injection
CRESEMBA (isavuconazonium sulfate) for injection is supplied as white to yellow sterile lyophilized powder containing 372 mg isavuconazonium sulfate (equivalent to 200 mg isavuconazole) in a single-dose vial and is packaged as follows:Individually packaged single-dose vial
NDC 0469-0420-01
Close16.2 Storage and Handling
CRESEMBA Capsules
Store CRESEMBA capsules at 20°C to 25°C (68°F to 77°F) in the original packaging to protect from moisture. Excursions are permitted from 15°C to 30°C (59°F to 86°F) [See USP Controlled Room Temperature].
CRESEMBA for Injection
Store CRESEMBA for injection unreconstituted vials at 2°C to 8°C (36°F to 46°F) in a refrigerator. CRESEMBA for injection is a single-dose vial of unpreserved sterile lyophile.
Following reconstitution of the lyophile with water for injection USP, the reconstituted solution should be used immediately, or stored between 5°C to 25°C (41°F to 77°F) for a maximum of 1 hour prior to preparation of the patient infusion solution [see Dosage and Administration (2.4)]. The prepared infusion solution should be kept for not more than 6 hours at room temperature [20°C to 25°C (68°F to 77°F)] or 24 hours at 2°C to 8°C (36°F to 46°F) prior to use [see Dosage and Administration (2.5)]. For nasogastric tube use, the reconstituted solution should be stored between 5°C to 25°C (41°F to 77°F) and used within 1 hour of reconstitution [see Dosage and Administration (2.6)].
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Important Administration Instructions - Advise patients that CRESEMBA can be taken with or without food. Each ...
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Important Administration Instructions
Advise patients that CRESEMBA can be taken with or without food. Each capsule should be swallowed whole. Do not chew, crush, dissolve, or open the capsules [see Dosage and Administration 2.1].
Drug Interactions
Advise patients to inform their physician if they are taking other drugs or before they begin taking other drugs as certain drugs can decrease or increase the plasma concentrations of CRESEMBA [see Warnings and Precautions (5.5)].
CRESEMBA can decrease or increase the plasma concentrations of other drugs [see Drug Interactions (7)].
Pregnancy
Advise patients to inform their physician if they are pregnant, plan to become pregnant, or are nursing [see Warnings and Precautions (5.4) and Pregnancy (8.1)].
Allergic Reactions
Advise patients to inform their physician immediately if they have ever had an allergic reaction to isavuconazole or other antifungal medicines such as ketoconazole, fluconazole, itraconazole, posaconazole, or voriconazole. Advise patients to discontinue CRESEMBA and seek immediate medical attention if any signs or symptoms of severe allergic reaction occur [see Warnings and Precautions (5.3)].
Marketed and Distributed by:
Astellas Pharma US, Inc.
Northbrook, IL 60062
CRESEMBA is a registered trademark of Astellas US LLC.Licensed from: Basilea Pharmaceutica International Ltd.
Close
390714-ISA-USA -
PATIENT PACKAGE INSERTPatient Information - CRESEMBA® (Crē sem’ bah) (isavuconazonium sulfate) capsules, for oral use - CRESEMBA® (Crē sem’ bah) (isavuconazonium sulfate) for injection, for intravenous ...Close
Patient Information
CRESEMBA® (Crē sem’ bah)
(isavuconazonium sulfate)
capsules, for oral use
CRESEMBA® (Crē sem’ bah)(isavuconazonium sulfate)
for injection, for intravenous use
Read this Patient Information before you or your child start taking CRESEMBA and each time you or your child get a refill. There may be new information. This information does not take the place of talking with your or your child’s healthcare provider about your medical condition or your treatment.
What is CRESEMBA?
CRESEMBA is a prescription medicine used to treat certain types of fungal infections in the blood or body called invasive aspergillosis and invasive mucormycosis as follows:
- •
- CRESEMBA for injection: adults and children 1 year of age and older
- •
- CRESEMBA capsules: adults and children 6 years of age and older who weigh 35 pounds (16 kg) or more
It is not known if CRESEMBA capsules are safe and effective in children younger than 6 years of age or who are 6 years of age and older but weigh less than 35 pounds (16 kg).
It is not known if CRESEMBA is safe and effective in children under 1 year of age.
Who should not take CRESEMBA?
Do not take CRESEMBA if you or your child:- •
- are allergic to CRESEMBA or any of the ingredients. See the end of this Patient Information leaflet for a complete list of ingredients in CRESEMBA.
- •
- are taking any of the following medicines:
- o
- ketoconazole
- o
- high-dose ritonavir
- o
- rifampin
- o
- carbamazepine
- o
- St. John’s wort (herbal supplement)
- o
- long-acting barbiturates
- •
- have a genetic problem that affects the electrical system of the heart (familial short QT syndrome).
Talk to your or your child’s healthcare provider or pharmacist if you are not sure if you are taking any of these medicines or have any of the conditions listed above.
Do not start taking new medicines without talking to your or your child’s healthcare provider or pharmacist.
Before you or your child take CRESEMBA, tell your healthcare provider about all of your medical conditions, including if you or your child:
- •
- have or ever had an abnormal heart rate or rhythm. Your healthcare provider may order a test to check your heart (ECG) before starting CRESEMBA.
- •
- have liver problems. Your healthcare provider may do blood tests to make sure you can take CRESEMBA.
- •
- have ever had an allergic reaction to other antifungal medicines such as ketoconazole, fluconazole, itraconazole, voriconazole or posaconazole.
- •
- are pregnant or plan to become pregnant. CRESEMBA may harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant. Women who can become pregnant should use effective birth control while taking CRESEMBA and for 28 days after the last CRESEMBA dose. Talk to your healthcare provider about birth control methods that may be right for you.
- •
- are breastfeeding or plan to breastfeed. CRESEMBA can pass into your breast milk and may harm your baby. Talk to your healthcare provider about the best way to feed your baby if you take CRESEMBA.
Tell your healthcare provider about all the medicines you or your child take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
CRESEMBA may affect the way other medicines work, and other medicines may affect how CRESEMBA works and can cause side effects.
Ask your or your child’s healthcare provider or pharmacist for a list of these medicines if you are not sure.
Know the medicines you or your child take. Keep a list of them with you to show your or your child’s healthcare provider and pharmacist when you get a new medicine.
How should I take CRESEMBA?
- •
- Take CRESEMBA exactly as your or your child’s healthcare provider tells you to take it.
- •
- Do not stop taking CRESEMBA until your or your child’s healthcare provider tells you to.
- •
- If you or your child take too much CRESEMBA, call your healthcare provider or go to the nearest hospital emergency room right away.
- •
- CRESEMBA capsules can be taken with or without food.
- •
- Swallow CRESEMBA capsules whole. Do not chew, crush, dissolve, or open the capsules.
- •
- CRESEMBA for injection can be given through a nasogastric tube by your or your child’s healthcare provider.
- •
- Do not give CRESEMBA capsules through a nasogastric tube.
Instructions on opening CRESEMBA capsules blister packaging:
CRESEMBA capsules are in child-resistant blister packaging.
Each blister section contains two pockets: one pocket for the CRESEMBA capsule and one pocket for the desiccant to protect the capsule from moisture (located to the left of the capsule).
- •
- Only open the blister packaging at time of use. Make sure only the CRESEMBA capsule pocket is opened.
- •
- Do not puncture the pocket containing the desiccant.
- •
- Do not swallow or use the desiccant.
What are the possible side effects of CRESEMBA?
CRESEMBA may cause serious side effects, including:
- •
- liver problems. Liver problems can happen in some people taking CRESEMBA. Some people who also have other serious medical problems may get severe liver problems which can lead to hepatitis, gallbladder problems, liver failure or death. Your or your child’s healthcare provider should do blood tests to check your liver before you start and while you are taking CRESEMBA. Call your or your child’s healthcare provider right away if you have any of the following symptoms of liver problems:
- o
- itchy skin
- o
- nausea or vomiting
- o
- yellowing of your eyes
- o
- feeling very tired
- o
- flu-like symptoms
- •
- infusion reactions. Infusion reactions can happen in people receiving CRESEMBA intravenously. If an infusion reaction happens, your or your child’s infusion will be stopped. Symptoms of an infusion reaction may include:
- o
- low blood pressure
- o
- difficulty breathing
- o
- chills
- o
- dizziness
- o
- numbness and tingling
- o
- changes in your sense of touch (hypoesthesia)
- •
- severe allergic reactions. Severe allergic reactions including death can happen in some people taking CRESEMBA. Symptoms of a severe allergic reaction may include:
- o
- swelling of your face, lips, mouth, or tongue
- o
- wheezing
- o
- skin rash redness, or swelling
- o
- fast heartbeat or pounding in your chest
- o
- trouble breathing
- o
- severe itching
- o
- dizziness or fainting
- o
- sweating
- Stop taking CRESEMBA and call your or your child’s healthcare provider or go to the nearest hospital emergency room right away if you have any of the symptoms listed above.
- •
- drug interactions with cyclosporine, sirolimus, or tacrolimus. If you or your child take CRESEMBA with cyclosporine, sirolimus, or tacrolimus, your blood levels of cyclosporine, sirolimus, or tacrolimus may increase. Serious side effects can happen in your or your child’s kidney or brain if you have high levels of cyclosporine, sirolimus, or tacrolimus in your blood. Your or your child’s healthcare provider should do blood tests to check your levels of cyclosporine, sirolimus, or tacrolimus if you are taking these medicines while taking CRESEMBA. Tell your or your child’s healthcare provider right away if you have swelling in your arm or leg or shortness of breath.
- •
- medicine interactions. Taking CRESEMBA with some other medicines may affect the way other medicines work causing serious side effects. Other medicines may affect the way CRESEMBA works, causing serious side effects. Tell your or your child’s healthcare provider about all the medicines you take.
The most common side effects of CRESEMBA in adults include:
- •
- nausea
- •
- vomiting
- •
- diarrhea
- •
- headache
- •
- changes in the level of a liver enzyme in your blood
- •
- low potassium
- •
- constipation
- •
- shortness of breath
- •
- cough
- •
- swelling of arms or legs
- •
- back pain
The most common side effects of CRESEMBA in children include:
- •
- diarrhea
- •
- stomach pain
- •
- vomiting
- •
- changes to the level of a liver enzyme in your blood
- •
- rash
- •
- nausea
- •
- itching
- •
- headache
Tell your or your child’s healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of CRESEMBA. For more information, ask your or your child’s healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store CRESEMBA capsules?
- •
- Store CRESEMBA at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- Keep CRESEMBA in the original package and protect it from moisture.
- •
- Do not remove CRESEMBA from its original packaging.
- •
- Safely throw away medicine that is out of date or no longer needed.
Keep CRESEMBA and all medicines out of the reach of children.
General information about the safe and effective use of CRESEMBA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use CRESEMBA for a condition for which it was not prescribed. Do not give CRESEMBA to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about CRESEMBA. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about CRESEMBA that is written for health professionals.
What are the ingredients of CRESEMBA capsules?
Active ingredient: isavuconazonium sulfate.
Inactive ingredients: black iron oxide, colloidal silicon dioxide, disodium edetate, gellan gum, hypromellose, magnesium citrate, microcrystalline cellulose, potassium acetate, potassium hydroxide, propylene glycol, purified water, red iron oxide, shellac, sodium lauryl sulfate, stearic acid, strong ammonia solution, talc and titanium dioxide.
Marketed and Distributed by:
Astellas Pharma US, Inc. Northbrook, IL 60062
390714-ISA- USA
CRESEMBA is a registered trademark of Astellas US LLC.
Licensed from: Basilea Pharmaceutica International Ltd.
For more information go to www.CRESEMBA.com or call 1-800-727-7003.
- This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 12/2023
-
Package/Label Display Panel – CRESEMBA capsules 186 mg – blister carton labelNDC 0469-0520-02 - CRESEMBA® (isavuconazonium sulfate) capsules - 186 mg* *equivalent to 100 mg isavuconazole - Unit dose blister - 14 Capsules - Rx Only
NDC 0469-0520-02
CRESEMBA®
(isavuconazonium sulfate) capsules186 mg*
*equivalent to 100 mg isavuconazole
Unit dose blister
14 CapsulesRx Only
Close -
Package/Label Display Panel - CRESEMBA for injection 372 mg – individual vial carton labelNDC 0469-0420-01 - CRESEMBA® (isavuconazonium sulfate) for injection - 372 mg* *equivalent to 200 mg isavuconazole - For Intravenous Infusion Only - Use an in-line filter during infusion - Carefully ...
NDC 0469-0420-01
CRESEMBA®
(isavuconazonium sulfate) for injection372 mg*
*equivalent to 200 mg isavuconazole
For Intravenous Infusion Only
Use an in-line filter during infusion
Carefully read the preparation and
administration instructions prior to useSingle Dose Vial
Discard unused portion
Rx Only
Close -
Package/Label Display Panel – CRESEMBA capsules 74.5 mg – blister carton label NDC 0469-2860-35 - CRESEMBA® (isavuconazonium sulfate) capsules - 74.5 mg* *equivalent to 40 mg isavuconazole - Unit dose blister - 35 Capsules - Rx Only
NDC 0469-2860-35
CRESEMBA®
(isavuconazonium sulfate) capsules74.5 mg*
*equivalent to 40 mg isavuconazole
Unit dose blister
35 CapsulesRx Only
Close -
INGREDIENTS AND APPEARANCEProduct Information
CRESEMBA isavuconazonium sulfate capsule Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0469-0520 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISAVUCONAZONIUM SULFATE (UNII: 31Q44514JV) (ISAVUCONAZOLE - UNII:60UTO373KE) ISAVUCONAZOLE 100 mg Product Characteristics Color ORANGE (Swedish orange (reddish brown color) body with white cap) Score no score Shape CAPSULE Size 24mm Flavor Imprint Code 766 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0469-0520-02 2 in 1 CARTON 11/04/2015 1 7 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA207500 11/04/2015 CRESEMBA isavuconazonium sulfate injection, powder, lyophilized, for solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0469-0420 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISAVUCONAZONIUM SULFATE (UNII: 31Q44514JV) (ISAVUCONAZOLE - UNII:60UTO373KE) ISAVUCONAZOLE 40.0 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0469-0420-01 1 in 1 CARTON 03/06/2015 1 5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA207501 03/06/2015 CRESEMBA isavuconazonium sulfate capsule Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0469-2860 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISAVUCONAZONIUM SULFATE (UNII: 31Q44514JV) (ISAVUCONAZOLE - UNII:60UTO373KE) ISAVUCONAZOLE 40 mg Product Characteristics Color ORANGE (Swedish orange (reddish brown color) body with orange cap) Score no score Shape CAPSULE Size 16mm Flavor Imprint Code 557 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0469-2860-35 7 in 1 CARTON 11/22/2022 1 5 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA207500 11/22/2022
CloseLabeler - Astellas Pharma US, Inc. (605764828)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
CRESEMBA- isavuconazonium sulfate capsule
CRESEMBA- isavuconazonium sulfate injection, powder, lyophilized, for solution
Number of versions: 20
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Mar 27, 2025 | 20 (current) | download |
| Mar 24, 2025 | 19 | download |
| Dec 23, 2024 | 18 | download |
| Jan 12, 2024 | 17 | download |
| Jan 12, 2023 | 16 | download |
| Dec 12, 2022 | 15 | download |
| May 18, 2022 | 14 | download |
| Mar 2, 2022 | 13 | download |
| Dec 20, 2021 | 12 | download |
| Jun 17, 2021 | 11 | download |
| Jun 3, 2021 | 10 | download |
| May 28, 2021 | 9 | download |
| Dec 31, 2019 | 8 | download |
| Jul 27, 2018 | 7 | download |
| Aug 11, 2016 | 6 | download |
| Nov 9, 2015 | 5 | download |
| Sep 2, 2015 | 4 | download |
| Aug 12, 2015 | 3 | download |
| Jun 4, 2015 | 2 | download |
| Apr 22, 2015 | 1 | download |
RxNorm
CRESEMBA- isavuconazonium sulfate capsule
CRESEMBA- isavuconazonium sulfate injection, powder, lyophilized, for solution
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 1608327 | isavuconazonium sulfate 186 MG Oral Capsule | PSN |
| 2 | 1608327 | isavuconazonium sulfate 186 MG Oral Capsule | SCD |
| 3 | 1608327 | isavuconazonium sulfate 186 MG (isavuconazole 100 MG) Oral Capsule | SY |
| 4 | 1608333 | Cresemba 186 MG Oral Capsule | PSN |
| 5 | 1608333 | isavuconazonium sulfate 186 MG Oral Capsule [Cresemba] | SBD |
| 6 | 1608333 | Cresemba 186 MG (isavuconazole 100 MG) Oral Capsule | SY |
| 7 | 1608333 | Cresemba 186 MG Oral Capsule | SY |
| 8 | 1608811 | isavuconazonium sulfate 372 Injection | PSN |
| 9 | 1608811 | isavuconazonium sulfate 372 MG Injection | SCD |
| 10 | 1608815 | Cresemba 372 MG Injection | PSN |
| 11 | 1608815 | isavuconazonium sulfate 372 MG Injection [Cresemba] | SBD |
| 12 | 1608815 | Cresemba 372 MG Injection | SY |
| 13 | 2623470 | isavuconazonium sulfate 74.5 MG Oral Capsule | PSN |
| 14 | 2623470 | isavuconazonium sulfate 74.5 MG Oral Capsule | SCD |
| 15 | 2623470 | isavuconazonium sulfate 74.5 MG (isavuconazole 40 MG) Oral Capsule | SY |
| 16 | 2623472 | Cresemba 74.5 MG Oral Capsule | PSN |
| 17 | 2623472 | isavuconazonium sulfate 74.5 MG Oral Capsule [Cresemba] | SBD |
| 18 | 2623472 | Cresemba 74.5 MG (isavuconazole 40 MG) Oral Capsule | SY |
| 19 | 2623472 | Cresemba 74.5 MG Oral Capsule | SY |
Get Label RSS Feed for this Drug
CRESEMBA- isavuconazonium sulfate capsule
CRESEMBA- isavuconazonium sulfate injection, powder, lyophilized, for solution
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=8f7f73b8-586a-4df0-935f-fecd4696c16c
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
CRESEMBA- isavuconazonium sulfate capsule
CRESEMBA- isavuconazonium sulfate injection, powder, lyophilized, for solution
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 0469-0420-01 |
| 2 | 0469-0520-02 |
| 3 | 0469-2860-35 |