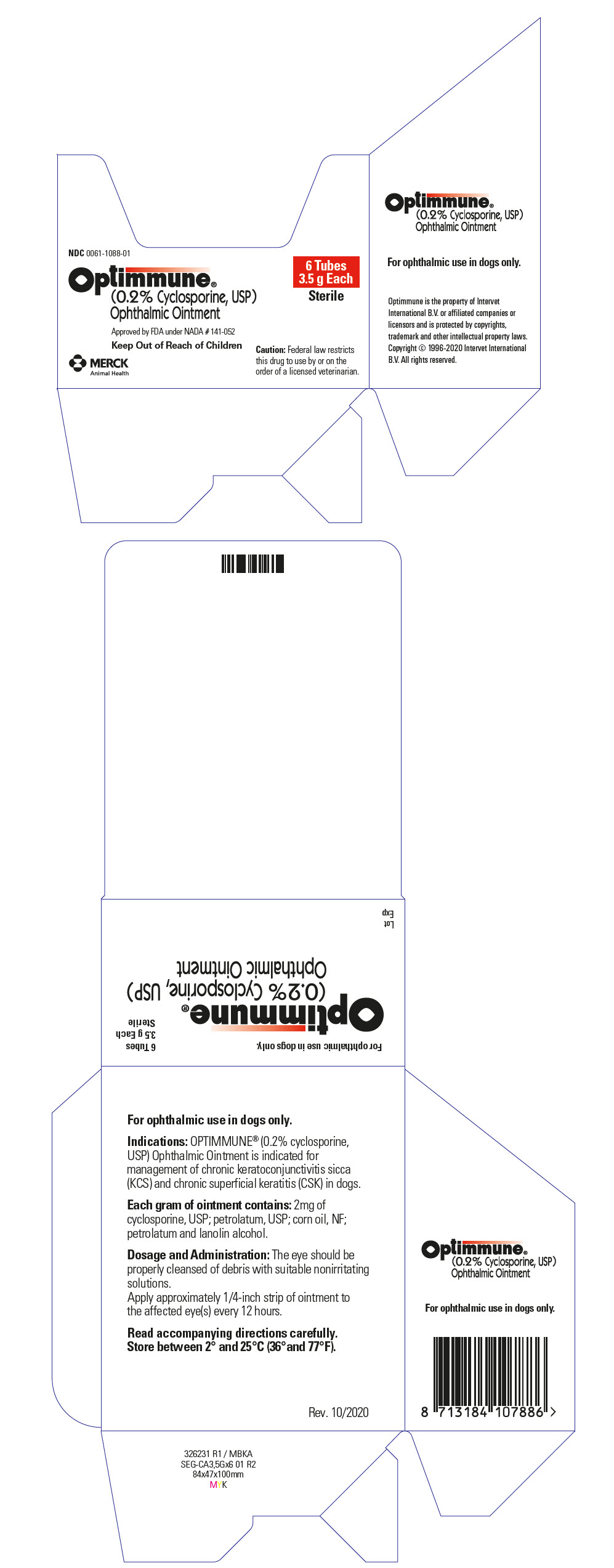
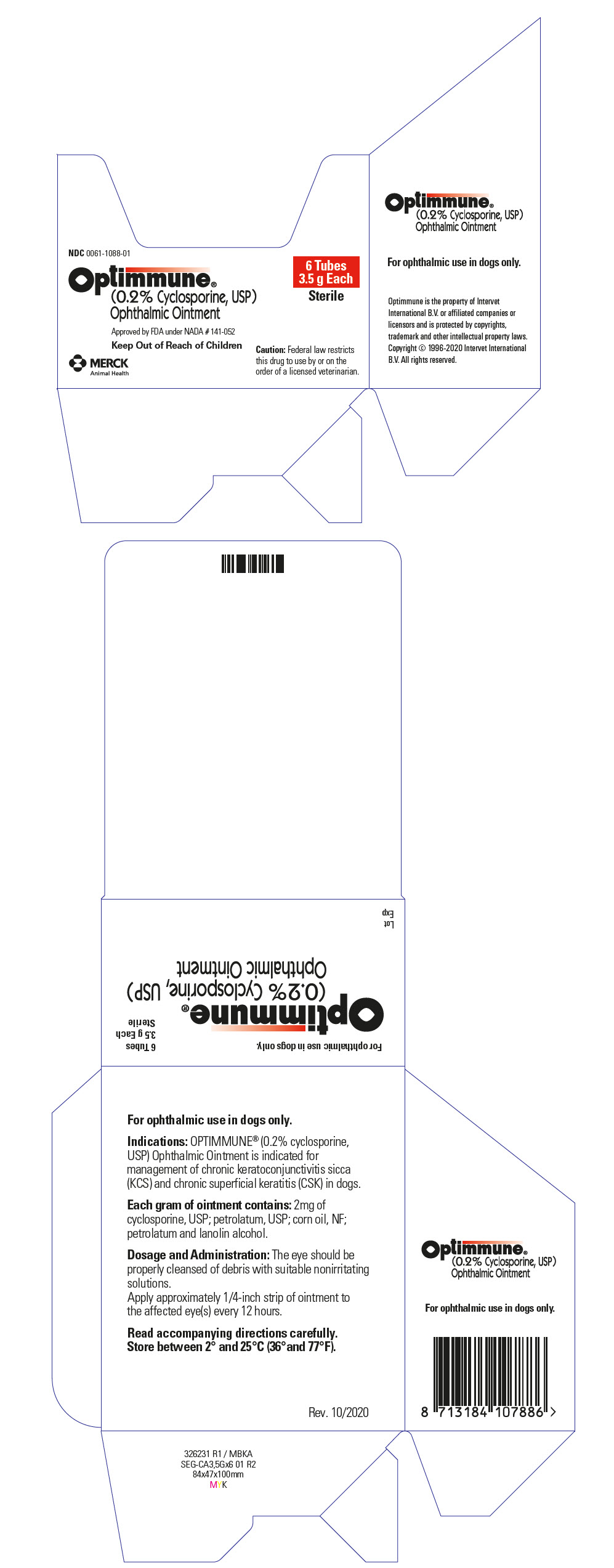
Label: OPTIMMUNE OPHTHALMIC- cyclosporine ointment
- NDC Code(s): 0061-1088-01
- Packager: Merck Sharp & Dohme Corp.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated November 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- CAUTION
-
DESCRIPTION
Each gram of OPTIMMUNE® Ophthalmic Ointment contains 2 mg of cyclosporine, USP; petrolatum, USP; corn oil, NF; petrolatum and lanolin alcohol. Cyclosporine (cyclosporin A), the active ingredient of OPTIMMUNE® Ophthalmic Ointment, is a cyclic undecapeptide metabolite of the fungus Tolypocladium inflatum gams.
-
MODE OF ACTION
When applied ophthalmically, cyclosporine is believed to act as a local immunomodulator of diseases suspected to be immune-mediated such as keratoconjunctivitis sicca (KCS) and chronic superficial keratitis (CSK). In the management of KCS, the mechanism by which cyclosporine causes an increase in lacrimation is poorly understood. Clinical improvement in cases of KCS is not necessarily dependent on an increase in aqueous tear production (as measured by the Schirmer Tear Test [STT]). See EFFICACY.
- INDICATIONS
-
PRECAUTIONS
The clinical effects of OPTIMMUNE® Ophthalmic Ointment have not been determined in dogs with KCS due to the following conditions: congenital alacrima, sulfonamide usage, canine distemper virus, metabolic disease, surgical removal of the third eyelid gland, and facial nerve paralysis with loss of the palpebral reflex. Some of the underlying conditions which may lead to KCS can be either transient (eg, facial nerve trauma) or correctable with appropriate treatment. Consequently, recovery from clinical signs attributed to KCS may be observed and treatment options may need reconsideration.
When switching to cyclosporine from another therapeutic agent (eg, frequent application of an artificial tear preparation) for KCS or CSK, it should be kept in mind that clinical efficacy is not necessarily apparent immediately after initiation of OPTIMMUNE® Ophthalmic Ointment therapy. Several days to a few weeks may be required before the clinical effects of OPTIMMUNE® Ophthalmic Ointment are of sufficient magnitude such that previously initiated therapy can be safely withdrawn. Abrupt cessation of a therapeutic agent immediately upon initiation of OPTIMMUNE® Ophthalmic Ointment therapy can result in rapid clinical relapse which may be erroneously interpreted as an adverse reaction to OPTIMMUNE® Ophthalmic Ointment.
The safety of OPTIMMUNE® Ophthalmic Ointment has not been determined in cases of preexisting viral or fungal ocular infections. It is recommended that in such cases, OPTIMMUNE® Ophthalmic Ointment therapy be delayed until the fungal/viral ocular infection has been successfully treated.
The safety of OPTIMMUNE® Ophthalmic Ointment in puppies, pregnant bitches, or dogs used for breeding has not been determined.
-
EFFICACY
1. KCS A well-controlled clinical field trial was conducted by veterinary ophthalmologists in 9 states and included 132 dogs afflicted with KCS of which 124 were evaluated for efficacy. Dogs were randomly assigned to BID treatment with either 0.2% (OPTIMMUNE® Ophthalmic Ointment) or 0% (placebo vehicle) cyclosporine ophthalmic ointment for 12 weeks. Treatment with OPTIMMUNE® Ophthalmic Ointment resulted in an average 8 to 9 mm increase in STT by the end of the study period (vs 3 to 4 mm for the placebo vehicle). Most of the increase in STT, approximately 6 mm, occurred in the first week of therapy. Some dogs improved clinically (ie, exhibited a decrease in conjunctival and/or corneal pathology) without an increase in STT values. This is thought to occur through suppression of inflammation by cyclosporine on the ocular surface. In this clinical field trial, OPTIMMUNE® Ophthalmic Ointment therapy was also associated with an improvement in clinical signs in comparison to the placebo. Blepharitis, blepharospasm, and "other signs of ocular discomfort" (eg, pawing at eyes), were markedly reduced. Improvement in conjunctival health as manifested by reduced conjunctival hypertrophy, reduced hyperemia, reduced conjunctival discharge volume, and improved character of discharge was evident. Improvement in corneal health as manifested by improved corneal surface contour, reduced corneal edema and corneal neovascularization was also noted. Overall improvement was noted in 81% of eyes treated with OPTIMMUNE® Ophthalmic Ointment.
Withdrawal of OPTIMMUNE® Ophthalmic Ointment therapy resulted in rapid clinical regression in all but one test eye indicating the need for long-term continual therapy for almost all cases of chronic KCS.
2. CSK The efficacy of OPTIMMUNE® Ophthalmic Ointment was determined in a historical controlled clinical field trial conducted by veterinary ophthalmologists in four countries and included 36 dogs afflicted with CSK. Dogs, primarily German shepherds, a breed disposed to CSK (German shepherd pannus), were treated twice daily with OPTIMMUNE® Ophthalmic Ointment for 6 weeks. Clinical improvement was noted by the investigators in 90.3% of eyes treated with OPTIMMUNE® Ophthalmic Ointment when compared to baseline.
-
SAFETY
A target animal safety study and clinical field studies with OPTIMMUNE® Ophthalmic Ointment showed a wide safety margin in adult dogs. In the 6-month target animal safety study, dogs were subjected twice daily to up to 10 times the approved concentration of OPTIMMUNE® Ophthalmic Ointment. No apparent toxicity or adverse reactions were observed. Dogs in this study were vaccinated with commercially available vaccines. No effect on antibody titer response was noted. Epiphora was noted in all groups, including the placebo group, and was not associated with any inflammatory change, nor was there any correlation to gross and histopathological changes.
-
ADVERSE REACTIONS
In the KCS clinical field trial, there were 20 adverse reactions reported out of 132 cases enrolled. This corresponds to an adverse reaction rate of 12.9% (13 of 101 cases) for OPTIMMUNE® Ophthalmic Ointment treated dogs and 22.6% (7 of 31) for placebo treated dogs. The reactions described were primarily ocular and periocular inflammatory reactions. These were likely a function of therapy being unable to fully control the keratoconjunctivitis, rather than a true "adverse reaction." Similarly, in the CSK trial, of 36 cases evaluated for safety, adverse reactions were noted in 2 animals (5.6%). One involved transient hyperemia, epiphora, and mild discomfort of the eye. The other involved periocular/palpebral inflammation and mild alopecia.
On rare occasion, instillation of OPTIMMUNE® Ophthalmic Ointment may be associated with local irritation as manifested by periocular redness, lid spasm, and excessive rubbing. As the eyes of dogs with KCS often demonstrate considerable inflammation, it will be difficult to determine whether this local irritation constitutes a hypersensitivity to OPTIMMUNE® Ophthalmic Ointment. If this ocular irritation persists beyond 7 days, hypersensitivity to a component of OPTIMMUNE® Ophthalmic Ointment should be suspected and therapeutic options reassessed.
-
DOSAGE AND ADMINISTRATION
Remove debris with suitable nonirritating solutions. Apply a 1/4 inch strip of
 ointment to the affected eye(s) every 12 hours. The ointment may be placed directly on the cornea or into the conjunctival sac.
ointment to the affected eye(s) every 12 hours. The ointment may be placed directly on the cornea or into the conjunctival sac.It is recommended that dogs exhibiting chronic recurring conjunctivitis be tested for adequate tear production to determine if they are suffering from early stages of chronic KCS.
For best results in treating KCS, cyclosporine ophthalmic ointment should be administered early in the course of the disease before irreversible damage to the lacrimal tissue, or dense corneal scarring or pigmentation occurs.
Dogs afflicted with KCS or CSK will most likely require lifelong consistent therapy (see EFFICACY section above). For CSK, because environmental factors such as ultraviolet (UV) radiation are implicated in the pathogenesis, clinical signs may subside in the winter months when light intensity is reduced or if the dog is moved to a lower altitude, or indoors, and thus exposed to less UV radiation.1
In cases refractory to cyclosporine, the diagnosis should be reevaluated and a different course of therapy considered. Periodic reassessment of the need for OPTIMMUNE® Ophthalmic Ointment therapy is recommended.
- HOW SUPPLIED
- REFERENCE
-
SPL UNCLASSIFIED SECTION
Formulated in France
Optimmune is the property of Intervet International B.V. or affiliated companies or licensors and is protected by copyrights, trademark and other intellectual property laws.
Copyright © 1996-2020 Intervet International B.V. All rights reserved.Rev. 10/2020
385398 R1Approved by FDA under NADA # 141-052
- PRINCIPAL DISPLAY PANEL - 3.5 g Tube Carton
-
INGREDIENTS AND APPEARANCE
OPTIMMUNE OPHTHALMIC
cyclosporine ointmentProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:0061-1088 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYCLOSPORINE (UNII: 83HN0GTJ6D) (CYCLOSPORINE - UNII:83HN0GTJ6D) CYCLOSPORINE 2 mg in 1 g Inactive Ingredients Ingredient Name Strength PARAFFIN (UNII: I9O0E3H2ZE) CORN OIL (UNII: 8470G57WFM) PETROLATUM (UNII: 4T6H12BN9U) LANOLIN ALCOHOLS (UNII: 884C3FA9HE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0061-1088-01 1 in 1 CARTON 1 3.5 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141052 08/26/1997 Labeler - Merck Sharp & Dohme Corp. (001317601) Establishment Name Address ID/FEI Business Operations TriRx SEGRE 274714834 MANUFACTURE Establishment Name Address ID/FEI Business Operations Teva Czech Industries s.r.o 643896244 API MANUFACTURE Establishment Name Address ID/FEI Business Operations Teva Pharmaceutical Works Private Limited Company 366709764 API MANUFACTURE Establishment Name Address ID/FEI Business Operations Teva Pharmaceutical Works Private Limited Company 366797490 API MANUFACTURE