Label: CALCIUM GLUCONATE injection, solution
- NDC Code(s): 0143-9185-01, 0143-9185-20
- Packager: Hikma Pharmaceuticals USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CALCIUM GLUCONATE INJECTION safely and effectively. See full prescribing information for CALCIUM GLUCONATE INJECTION.
CALCIUM GLUCONATE injection, for intravenous use
Initial U.S. Approval: 1941
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
• Contains 100 mg of calcium gluconate per mL which contains 9.3 mg (0.465 mEq) of elemental calcium (2.1)
• Administer intravenously (bolus or continuous infusion) via a secure intravenous line (2.1)
• See Full Prescribing Information (FPI) for dilution instructions, administration rates, and appropriate monitoring (2.1)
• Individualize the dose within the recommended range in adults and pediatric patients depending on the severity of symptoms of hypocalcemia, the serum calcium level, and the acuity of onset of hypocalcemia. See Table 1 in the FPI for dosing recommendations in mg of calcium gluconate for neonates, pediatric and adult patients (2.2)
• Measure serum calcium during intermittent infusions every 4 to 6 hours and during continuous infusion every 1 to 4 hours (2.3)
• Calcium Gluconate Injection is not physically compatible with fluids containing phosphate or bicarbonate. Precipitation may result if mixed. See FPI for all drug incompatibilities (2.5)
• Supplied in a pharmacy bulk package (PBP). For PBP, dispense single doses to many patients in a pharmacy admixture program; use within 4 hours of puncture (2.6)
DOSAGE FORMS AND STRENGTHS
Injection: (3)
• Pharmacy bulk package: 10,000 mg per 100 mL (100 mg per mL)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
• Arrhythmias with Concomitant Cardiac Glycoside Use If concomitant therapy is necessary, Calcium Gluconate Injection should be given slowly in small amounts and close ECG monitoring is recommended (5.1)
• End-Organ Damage due to Intravascular Ceftriaxone-Calcium Precipitates Concurrent use of intravenous ceftriaxone may cause life-threatening precipitates. Cases of fatal outcomes in neonates have occurred. (4, 5.2)
• Tissue Necrosis and Calcinosis Calcinosis cutis can occur with or without extravasation of Calcium Gluconate Injection. Tissue necrosis, ulceration, and secondary infection are the most serious complications. If extravasation occurs or clinical manifestations of calcinosis cutis are noted, immediately discontinue intravenous administration at that site and treat as needed. (5.3)
• Hypotension, Bradycardia, and Cardiac Arrhythmias with Rapid Administration To avoid adverse reactions that may follow rapid intravenous administration, Calcium Gluconate Injection should be diluted with 5% dextrose or normal saline and infused slowly, with careful ECG monitoring for cardiac arrhythmias. (5.4)
• Aluminum Toxicity This product contains aluminum, up to 400 mcg per liter, that may be toxic. (5.5)
ADVERSE REACTIONS
The most common adverse events with Calcium Gluconate Injection are local soft tissue inflammation and necrosis, calcinosis cutis and calcification that are related to extravasation. Other adverse events include vasodilation, decreased blood pressure, bradycardia, cardiac arrhythmia, syncope, and cardiac arrest. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Hikma Pharmaceuticals USA Inc. at 1-877-845-0689 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
• Cardiac Glycoside: Synergistic arrhythmias may occur if calcium and cardiac glycosides are administered together. (7.1)
• Calcium Channel Blockers: Administration of calcium may reduce the response. (7.2)
• Drugs that may cause hypercalcemia Vitamin D, vitamin A, thiazide diuretics, estrogen, calcipotriene and teriparatide administration may cause hypercalcemia. Monitor plasma calcium concentrations in patients taking these drugs concurrently. (7.3)USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Recommended Dosage
2.3 Serum Calcium Monitoring
2.4 Dosage in Renal Impairment
2.5 Drug Incompatibilities
2.6 Preparation of Pharmacy Bulk Package
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Arrhythmias with Concomitant Cardiac Glycoside Use
5.2 End-Organ Damage due to Intravascular Ceftriaxone-Calcium Precipitates
5.3 Tissue Necrosis and Calcinosis
5.4 Hypotension, Bradycardia, and Cardiac Arrhythmias with Rapid Administration
5.5 Aluminum Toxicity
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Cardiac Glycosides
7.2 Calcium Channel Blockers
7.3 Drugs that may cause Hypercalcemia
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
• Calcium Gluconate Injection contains 100 mg of calcium gluconate per mL which contains 9.3 mg (i.e., 0.465 mEq) of elemental calcium.
• Dilute Calcium Gluconate Injection prior to use in 5% dextrose or normal saline and assess for potential drug or IV fluid incompatibilities [see Dosage and Administration (2.5)].
• Inspect Calcium Gluconate Injection visually prior to administration. The solution should appear clear and colorless to slightly yellow. Do not administer if there is particulate matter or discoloration.
• Use the diluted solution immediately after preparation.
• Administer Calcium Gluconate Injection intravenously via a secure intravenous line to avoid calcinosis cutis and tissue necrosis [see Warnings and Precautions (5.3)].
• Administer Calcium Gluconate Injection by bolus administration or continuous infusion:
For bolus intravenous administration:
• Dilute the dose [see Dosage and Administration (2.2)] of Calcium Gluconate Injection in 5% dextrose or normal saline to a concentration of 10-50 mg/mL prior to administration. Administer the dose slowly and DO NOT exceed an infusion rate of 200 mg/minute in adults or 100 mg/minute in pediatric patients, including neonates. Monitor patients, vitals and electrocardiograph (ECG) during administration [see Warnings and Precautions (5.4)].
For continuous intravenous infusion:
• Dilute Calcium Gluconate Injection in 5% dextrose or normal saline to a concentration of 5.8-10 mg/mL prior to administration. Administer at the rate recommended in Table 1 [see Dosage and Administration (2.2)] and monitor patients, vitals, calcium and ECG during the infusion [see Warnings and Precautions (5.4)].
•Calcium Gluconate Injection is supplied in pharmacy bulk packages [see Dosage and Administration (2.6)].
2.2 Recommended Dosage
Individualize the dose of Calcium Gluconate Injection within the recommended range depending on the severity of symptoms of hypocalcemia, the serum calcium level, and the acuity of onset of hypocalcemia.
Table 1 provides dosing recommendations for Calcium Gluconate Injection in mg of calcium gluconate for neonates, pediatric and adult patients.
Table 1. Dosing Recommendations in mg of Calcium Gluconate for Neonate, Pediatric, and Adult Patients
Patient Population Initial Dose
Subsequent Doses (if needed)Bolus Continuous Infusion Neonate
(≤ 1 month)100 - 200 mg/kg 100 - 200 mg/kg
every 6 hoursInitiate at
17-33 mg/kg/hourPediatric
(> 1 month to
< 17 years)29 - 60 mg/kg 29 - 60 mg/kg
every 6 hoursInitiate at
8-13 mg/kg/hourAdult 1000 - 2000 mg 1000 - 2000 mg
every 6 hoursInitiate at
5.4 - 21.5 mg/kg/hourFor bolus administration, DO NOT exceed an infusion rate of:
• 200 mg/minute in adult patients
• 100 mg/minute in pediatric patients
For continuous infusions, adjust rate as needed based on serum calcium levels2.3 Serum Calcium Monitoring
Measure serum calcium every 4 to 6 hours during intermittent infusions with Calcium Gluconate Injection and measure serum calcium every 1 to 4 hours during continuous infusion.
2.4 Dosage in Renal Impairment
For patients with renal impairment, initiate Calcium Gluconate Injection at the lowest dose of the recommended dose ranges for all age groups and monitor serum calcium levels every 4 hours.
2.5 Drug Incompatibilities
- Do not mix Calcium Gluconate Injection with ceftriaxone. Concurrent use of intravenous ceftriaxone and Calcium Gluconate Injection can lead to the formation of ceftriaxone-calcium precipitates. Concomitant use of ceftriaxone and intravenous calcium-containing products is contraindicated in neonates (28 days of age or younger) [see Contraindications (4)]. In patients older than 28 days of age, ceftriaxone and calcium-containing products may be administered sequentially, provided the infusion lines are thoroughly flushed between infusions with a compatible fluid. Ceftriaxone must not be administered simultaneously with intravenous calcium-containing solutions via a Y-site in any age group [see Warnings and Precautions (5.2), Drug Interactions (7.3)].
- Do not mix Calcium Gluconate Injection with fluids containing bicarbonate or phosphate. Calcium Gluconate Injection is not physically compatible with fluids containing phosphate or bicarbonate. Precipitation may result if mixed.
- Do not mix Calcium Gluconate Injection with minocycline injection. Calcium complexes minocycline rendering it inactive.
2.6 Preparation of Pharmacy Bulk Package
The pharmacy bulk package (PBP) of Calcium Gluconate Injection is intended for dispensing of single doses to multiple patients in a pharmacy admixture program. Penetrate the container closure only one time with a suitable sterile transfer device or dispensing set that allows measured dispensing of the contents. Use the PBP only in a suitable ISO Class 5 work area such as a laminar flow hood (or an equivalent clean air compounding area). Complete dispensing from the pharmacy bulk vial within 4 hours after the container closure is penetrated. Each dose dispensed from the Pharmacy Bulk Package vial must be used immediately.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Calcium Gluconate Injection is contraindicated in:
• Hypercalcemia
• Neonates (28 days of age or younger) receiving ceftriaxone [see Warnings and Precautions (5.2)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Arrhythmias with Concomitant Cardiac Glycoside Use
Cardiac arrhythmias may occur if calcium and cardiac glycosides are administered together. Hypercalcemia increases the risk of digoxin toxicity. Administration of Calcium Gluconate Injection should be avoided in patients receiving cardiac glycosides. If concomitant therapy is necessary, Calcium Gluconate Injection should be given slowly in small amounts and with close ECG monitoring [see Drug Interactions (7.1)].
5.2 End-Organ Damage due to Intravascular Ceftriaxone-Calcium Precipitates
Concomitant use of ceftriaxone and Calcium Gluconate Injection is contraindicated in neonates (28 days of age or younger) due to cases of fatal outcomes in neonates in which a crystalline material was observed in the lungs and kidneys at autopsy after ceftriaxone and calcium were administrated simultaneously through the same intravenous line. Concomitant administration can lead to the formation of ceftriaxone-calcium precipitates that may act as emboli, resulting in vascular spasm or infarction [see Contraindications (4)].
In patients older than 28 days of age, ceftriaxone and Calcium Gluconate Injection may be administered sequentially, provided the infusion lines are thoroughly flushed between infusions with a compatible fluid. Do not administer Ceftriaxone simultaneously with Calcium Gluconate Injection via a Y-site in any age group.
5.3 Tissue Necrosis and Calcinosis
Intravenous administration of Calcium Gluconate Injection and local trauma may result in calcinosis cutis due to transient increase in local calcium concentration. Calcinosis cutis can occur with or without extravasation of Calcium Gluconate Injection, is characterized by abnormal dermal deposits of calcium salts, and clinically manifests as papules, plaques, or nodules that may be associated with erythema, swelling, or induration. Tissue necrosis, ulceration, and secondary infection are the most serious complications.
If extravasation occurs or clinical manifestations of calcinosis cutis are noted, immediately discontinue intravenous administration at that site and treat as needed.5.4 Hypotension, Bradycardia, and Cardiac Arrhythmias with Rapid Administration
Rapid injection of Calcium Gluconate Injection may cause vasodilation, decreased blood pressure, bradycardia, cardiac arrhythmias, syncope and cardiac arrest. To avoid adverse reactions that may follow rapid intravenous administration, Calcium Gluconate Injection should be diluted with 5% dextrose or normal saline and infused slowly. If rapid intravenous bolus of Calcium Gluconate Injection is required, the rate of intravenous administration should not exceed 200 mg/minute in adults and 100 mg/minute in pediatric patients and ECG monitoring during administration is recommended [see Dosage and Administration (2.1)].
5.5 Aluminum Toxicity
Calcium Gluconate Injection contains aluminum, up to 400 mcg per liter, that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum. Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 mcg/kg/day to 5 mcg/kg/day accumulate aluminum levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are also described elsewhere in the labeling:
• Arrhythmias with Concomitant Cardiac Glycoside Use [see Warnings and Precautions (5.1)]
• End-Organ Damage due to Intravascular Ceftriaxone-Calcium Precipitates [see Warnings and Precautions (5.2)]
• Tissue Necrosis and Calcinosis [see Warnings and Precautions (5.3)]
• Hypotension, Bradycardia, and Cardiac Arrhythmias [see Warnings and Precautions (5.4)]
• Aluminum toxicity [see Warnings and Precautions (5.5)]
The following adverse reactions associated with the use of calcium gluconate were identified in the literature. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or to establish a causal relationship to drug exposure.
Cardiovascular: Vasodilation, decreased blood pressure, bradycardia, cardiac arrhythmia, syncope, cardiac arrest
Administration site reactions: Local soft tissue inflammation, local necrosis, calcinosis cutis and calcification due to extravasation
-
7 DRUG INTERACTIONS
7.1 Cardiac Glycosides
Hypercalcemia increases the risk of digoxin toxicity, while digoxin may be therapeutically ineffective in the presence of hypocalcemia. Synergistic arrhythmias may occur if calcium and cardiac glycosides are administered together. Avoid administration of Calcium Gluconate Injection in patients receiving cardiac glycosides; if considered necessary, administer Calcium Gluconate Injection slowly in small amounts and monitor ECG closely during administration.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk summary
Limited available data with Calcium Gluconate Injection use in pregnant women are insufficient to inform a drug associated risk of adverse developmental outcomes. There are risks to the mother and the fetus associated with hypocalcemia in pregnancy [see Clinical Considerations].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated maternal risk
Maternal hypocalcemia can result in an increased rate of spontaneous abortion, premature and dysfunctional labor, and possibly preeclampsia.Fetal/Neonatal adverse reactions
Infants born to mothers with hypocalcemia can have associated fetal and neonatal hyperparathyroidism, which in turn can cause fetal and neonatal skeletal demineralization, subperiosteal bone resorption, osteitis fibrosa cystica and neonatal seizures. Infants born to mothers with hypocalcemia should be carefully monitored for signs of hypocalcemia or hypercalcemia, including neuromuscular irritability, apnea, cyanosis and cardiac rhythm disorders.8.2 Lactation
Risk summary
Calcium is present in human milk as a natural component of human milk. It is not known whether intravenous administration of Calcium Gluconate Injection can alter calcium concentration in human milk. There are no data on the effects of Calcium Gluconate Injection on the breastfed infant, or on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Calcium Gluconate Injection and any potential adverse effects on the breastfed child from Calcium Gluconate Injection or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of Calcium Gluconate Injection have been established in pediatric patients for the treatment of acute, symptomatic hypocalcemia.
Pediatric approval for Calcium Gluconate Injection, including doses, is not based on adequate and well-controlled clinical studies. Safety and dosing recommendations in pediatric patients are based on published literature and clinical experience [see Dosage and Administration (2.2)].
Concomitant use of ceftriaxone and Calcium Gluconate Injection is contraindicated in neonates (28 days of age or younger) due to reports of fatal outcomes associated with the presence of lung and kidney ceftriaxone-calcium precipitates. In patients older than 28 days of age, ceftriaxone and Calcium Gluconate Injection may be administered sequentially, provided the infusion lines are thoroughly flushed between infusions with a compatible fluid [see Contraindications (4) and Warnings and Precautions (5.2)]. This product contains up to 400 mcg/L aluminum which may be toxic, particularly for premature neonates due to immature renal function. Parenteral administration of aluminum greater than 4 to 5 mcg/kg/day is associated with central nervous system and bone toxicity [see Warnings and Precautions (5.5)].8.5 Geriatric Use
In general dose selection for an elderly patient should start at the lowest dose of the recommended dose range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Renal Impairment
For patients with renal impairment, initiate Calcium Gluconate Injection at the lowest dose of the recommended dose ranges across all age groups. Monitor serum calcium levels every 4 hours [see Dosage and Administration (2.4)].
-
10 OVERDOSAGE
Overdosage of Calcium Gluconate Injection may result in hypercalcemia. Symptoms of hypercalcemia typically develop when the total serum calcium concentration is ≥12 mg/dL. Neurologic symptoms include depression, weakness, fatigue, and confusion at lower levels, with patients experiencing hallucinations, disorientation, hypotonicity, seizures, and coma. Effects on the kidney include diminished ability to concentrate urine and diuresis.
If overdose of Calcium Gluconate Injection occurs immediately discontinue administration and provide supportive treatments to restore intravascular volume as well as promote calcium excretion in the urine if necessary. -
11 DESCRIPTION
Calcium Gluconate Injection, USP is a clear, colorless to slightly yellow, sterile, preservative-free, nonpyrogenic, supersaturated solution of calcium gluconate, a form of calcium, for intravenous use.
Calcium Gluconate is calcium D-gluconate (1:2) monohydrate. The structural formula is:
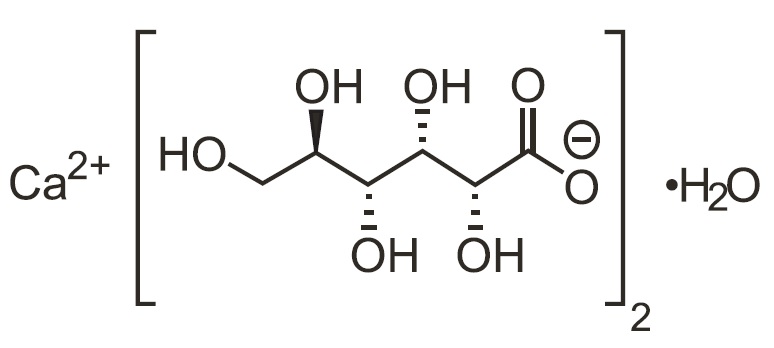
Molecular formula: C12H22CaO14• H2O
Molecular weight: 448.39
Solubility in water: 3.5 g/100 mL at 25°C
Calcium Gluconate Injection, USP is available as 10,000 mg per 100 mL (100 mg per mL) in a pharmacy bulk package.
Each mL of Calcium Gluconate Injection, USP contains 100 mg of calcium gluconate (equivalent to 94 mg of calcium gluconate and 4.5 mg of calcium saccharate tetrahydrate), hydrochloric acid and/or sodium hydroxide for pH adjustment (6.0 to 8.2) and water for injection, q.s. It contains no antimicrobial agent.
Each mL of Calcium Gluconate Injection, USP contains 9.3 mg (0.465 mEq) of elemental calcium.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Intravenous administration of calcium gluconate increases serum ionized calcium level. Calcium gluconate dissociates into ionized calcium in plasma. Ionized calcium and gluconate are normal constituents of body fluids.
12.3 Pharmacokinetics
Absorption
Calcium Gluconate Injection is 100% bioavailable following intravenous injection.
Metabolism
Calcium itself does not undergo direct metabolism. The release of ionized calcium from intravenous administration of calcium gluconate is direct and does not seem to be affected by the first pass through the liver.
Distribution
Calcium in the body is distributed mainly in skeleton (99%). Only 1% of the total body calcium is distributed within the extracellular fluids and soft tissues. About 50% of total serum calcium is in the ionized form and represents the biologically active part. 8% to 10% serum calcium is bound to organic and inorganic acid and approximately 40% is protein-bound (primarily to albumin).
Elimination
Studies have shown a relationship between urinary calcium excretion and the intravenous administration of calcium gluconate, with a significant increase in urinary calcium excretion observed after the intravenous administration of calcium gluconate.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been conducted to evaluate the carcinogenic potential of Calcium Gluconate Injection. Calcium gluconate was not mutagenic with or without metabolic activation in the Ames test with Salmonella typhimurium (strains TA-1535, TA-1537, and TA-1538) or Saccharomyces cerevisiae (Strain D4). Fertility studies in animals have not been conducted with calcium gluconate administered by the intravenous route.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Calcium Gluconate Injection, USP is a clear, colorless to slightly yellow solution free from visible particles supplied as follows:
NDC Strength/Vial Size (mL) 0143-9185-01 10,000 mg calcium gluconate per 100 mL (100 mg per mL),
in a 100 mL plastic, Pharmacy Bulk Package vial.0143-9185-20 10,000 mg calcium gluconate per 100 mL (100 mg per mL),
in a 100 mL plastic, Pharmacy Bulk Package vial, packaged in a carton of 20.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Do not freeze.
Preservative Free. Discard any unused portion in the Pharmacy Bulk Package vial within 4 hours after initial closure puncture.
Each dose dispensed from the Pharmacy Bulk Package vial must be used immediately.
The diluted solution must be used immediately.
NOTE: Supersaturated solutions are prone to precipitation. The precipitate, if present, may be dissolved by warming the vial to 60° to 80°C, with occasional agitation, until the solution becomes clear. Shake vigorously. Allow to cool to room temperature before dispensing. Use injection only if clear immediately prior to use. -
17 PATIENT COUNSELING INFORMATION
• Advise the patient that the risks associated with infusion including local tissue inflammation, local necrosis and calcinosis. [see Warnings and Precautions (5.3)].
Manufactured for:
Nivagen Pharmaceuticals, Inc.
Sacramento, CA 95827 USA
Distributed by:
Hikma Pharmaceuticals USA Inc.
Berkley Heights, NJ 07922
Rev. 09/2023 -
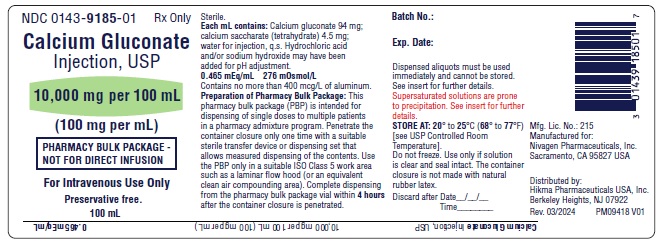
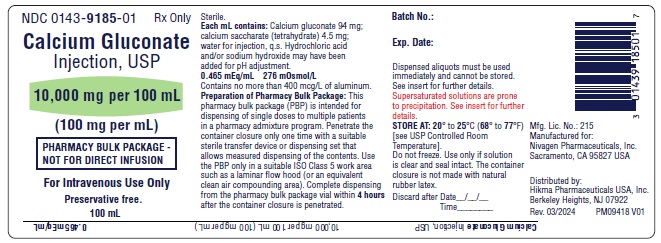
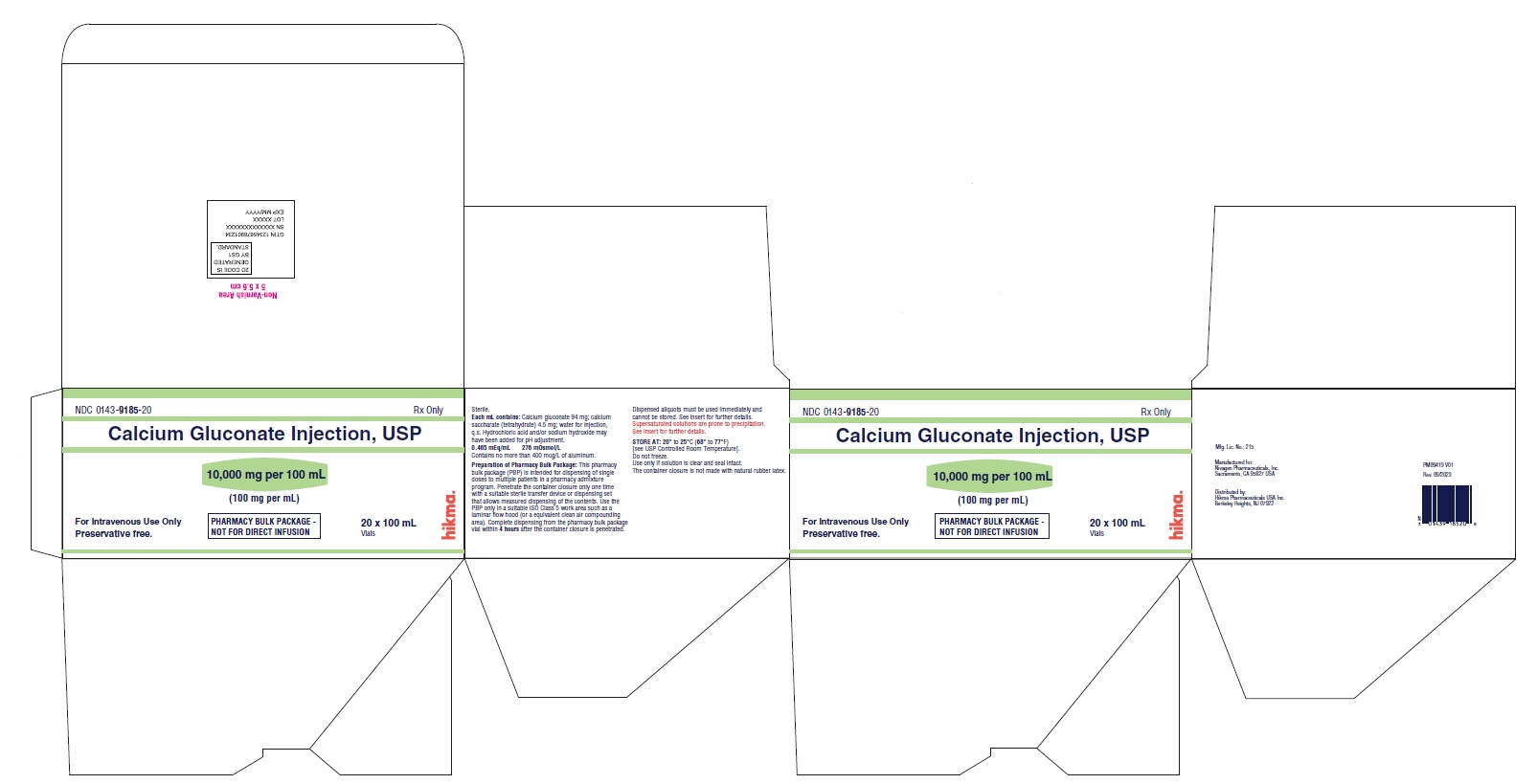
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Pharmacy Bulk Package Vial Label
NDC 0143-9185-01
Calcium Gluconate Injection, USP
10,000 mg per 100 mL
(100 mg per mL)
PHARMACY BULK PACKAGE – NOT FOR DIRECT INFUSION
Preservative free.
100 mL Rx only
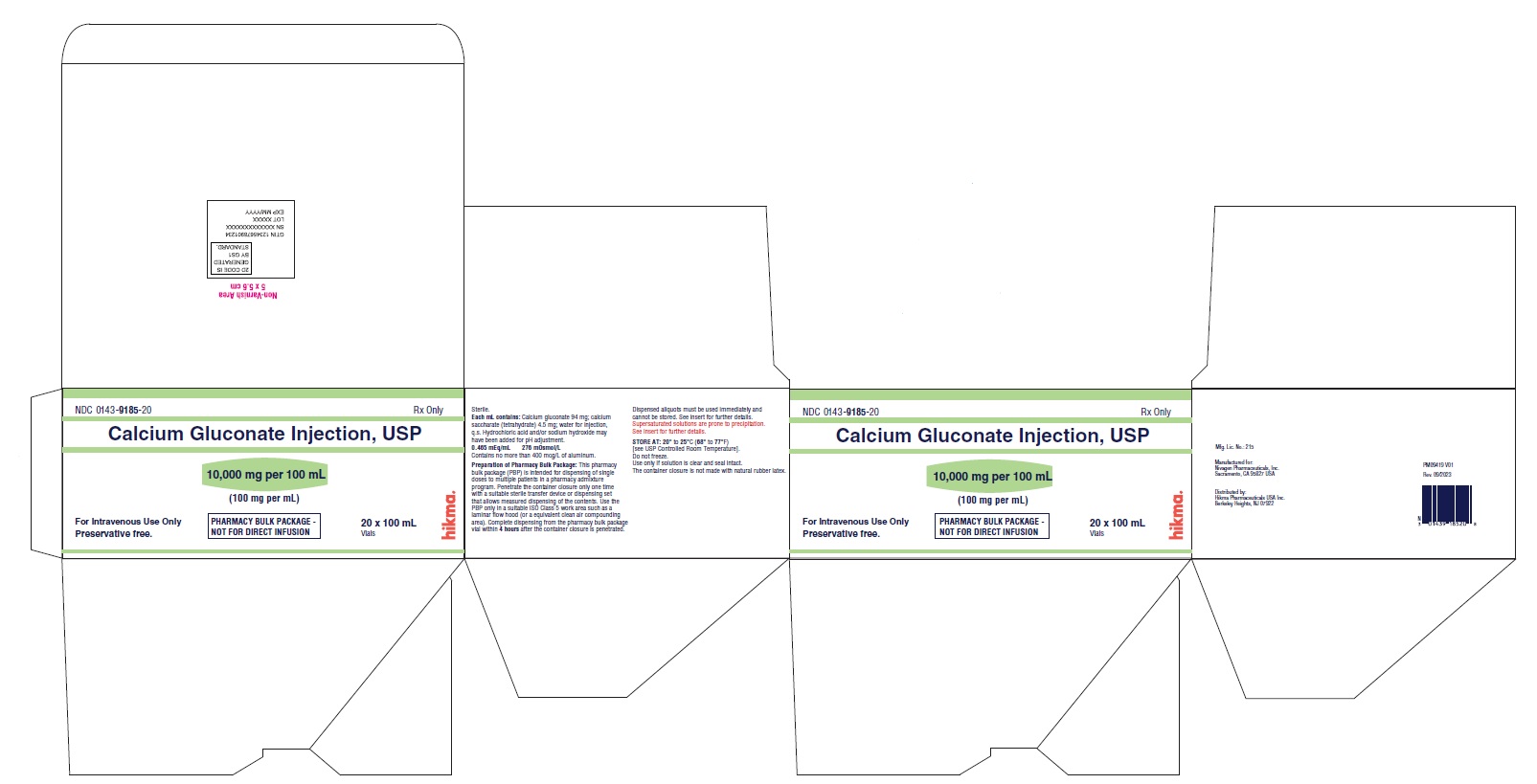
Pharmacy Bulk Package Carton Label
NDC 0143-9185-20
Calcium Gluconate Injection, USP
10,000 mg per 100 mL
(100 mg per mL)
PHARMACY BULK PACKAGE – NOT FOR DIRECT INFUSION
Preservative free.
20 x 100 mL
Vials Rx only
-
INGREDIENTS AND APPEARANCE
CALCIUM GLUCONATE
calcium gluconate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0143-9185 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM GLUCONATE MONOHYDRATE (UNII: CZN0MI5R31) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM GLUCONATE MONOHYDRATE 98 mg in 1 mL Inactive Ingredients Ingredient Name Strength CALCIUM SACCHARATE (UNII: 6AP9J91K4V) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0143-9185-20 20 in 1 CARTON 06/30/2024 1 NDC:0143-9185-01 100 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213071 06/30/2024 Labeler - Hikma Pharmaceuticals USA Inc. (001230762) Registrant - Nivagen Pharmaceuticals Inc (118903906)