Label: NICARDIPINE HYDROCHLORIDE- nicardipine hydrochloride injection
- NDC Code(s): 42571-394-88, 42571-394-89
- Packager: Micro Labs Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 23, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NICARDIPINE HYDROCHLORIDE INJECTION safely and effectively. See full prescribing information for NICARDIPINE HYDROCHLORIDE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Hypertension - Nicardipine hydrochloride injection is indicated for the short-term treatment of hypertension when oral therapy is not feasible or desirable. For prolonged control of blood ...
-
2 DOSAGE AND ADMINISTRATION2.1 General Information - Individualize dosing based on the severity of hypertension and the response of the patient during dosing. Monitor blood pressure and heart rate both during and after the ...
-
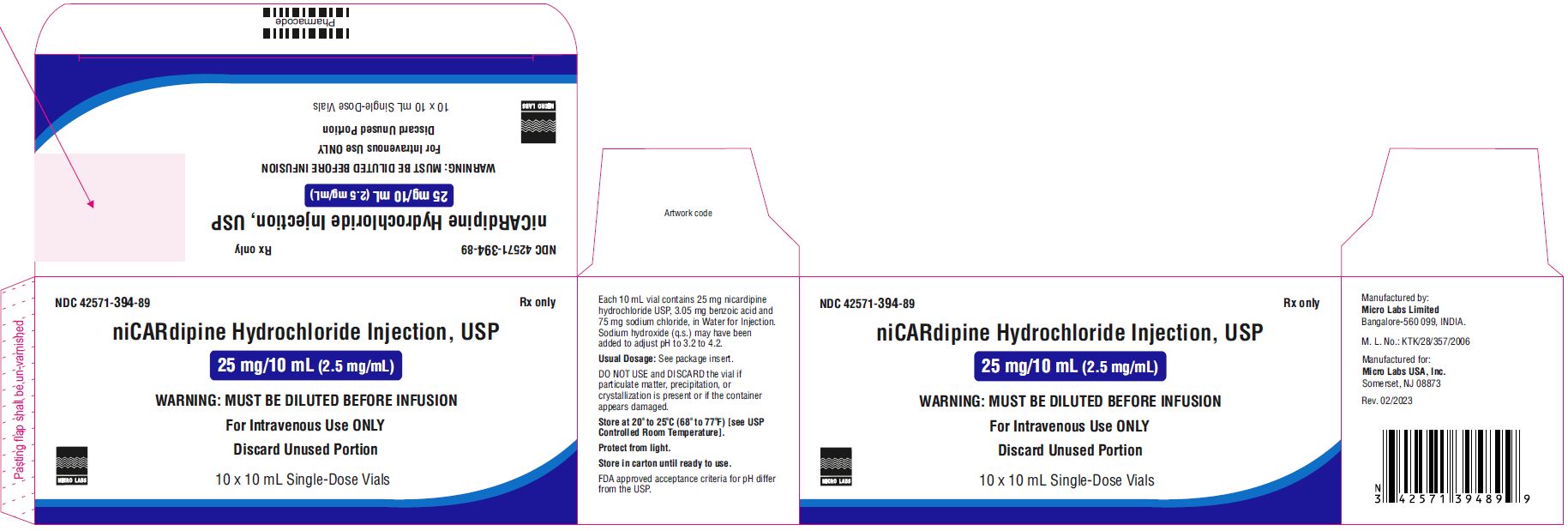
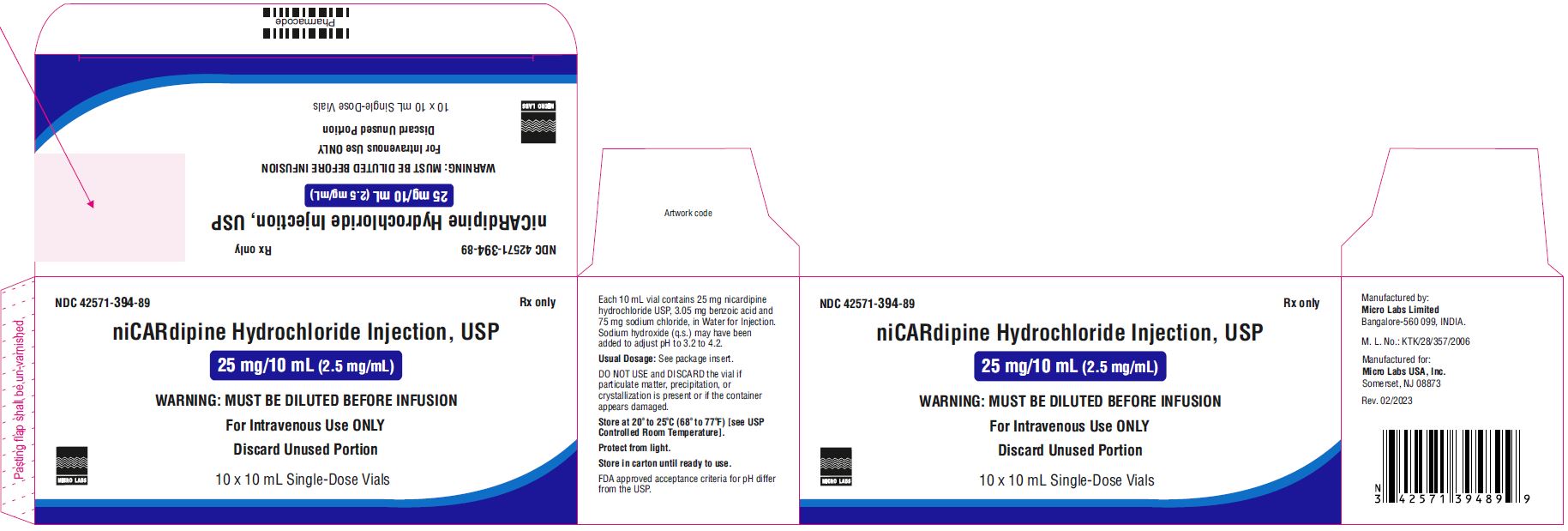
3 DOSAGE FORMS AND STRENGTHSNicardipinehydrochloride injection, USP is a clear, yellow color solution and is available in the following presentations: 25 mg nicardipine hydrochloride, USP in 10 mL injection (2.5 mg/mL) in ...
-
4 CONTRAINDICATIONS4.1 Advanced Aortic Stenosis - Do not use nicardipine in patients with advanced aortic stenosis because of the afterload reduction effect of nicardipine. Reduction of diastolic pressure in these ...
-
5 WARNINGS AND PRECAUTIONS5.1 Excessive Pharmacologic Effects - In administrating nicardipine, close monitoring of blood pressure and heart rate is required. Nicardipine may occasionally produce symptomatic hypotension or ...
-
6 ADVERSE REACTIONS6.1 Adverse Reactions Observed in Clinical Trials - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot ...
-
7 DRUG INTERACTIONS7.1 Antihypertensive Agents - Since nicardipine hydrochloride injection may be administered to patients already being treated with other medications, including other antihypertensive agents ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - There are no adequate and well-controlled studies of nicardipine use in pregnant women. There are limited human data in pregnant women with pre-eclampsia and preterm labor. In ...
-
10 OVERDOSAGESeveral overdosages with orally administered nicardipine have been reported. One adult patient allegedly ingested 600 mg of nicardipine immediate release capsules, and another patient, 2160 mg of ...
-
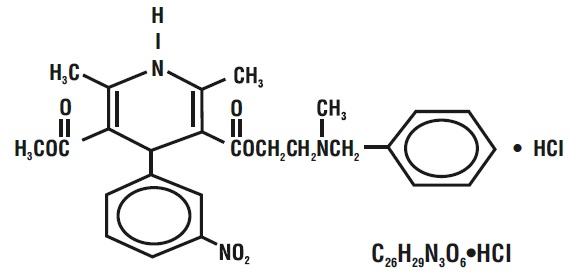
11 DESCRIPTIONNicardipine hydrochloride, USP is a calcium ion influx inhibitor (slow channel blocker or calcium channel blocker). Nicardipine hydrochloride injection, USP for intravenous administration contains ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Nicardipine inhibits the transmembrane influx of calcium ions into cardiac muscle and smooth muscle without changing serum calcium concentrations. The contractile ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Rats treated with nicardipine in the diet (at concentrations calculated to provide daily dosage levels of 5, 15, or 45 mg/kg/day) for ...
-
14 CLINICAL STUDIESEffects in Hypertension - In patients with mild-to-moderate chronic stable essential hypertension, nicardipine hydrochloride injection (0.5 to 4 mg/hr) produced dose-dependent decreases in ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Nicardipine Hydrochloride Injection, USP is supplied as a sterile, clear, yellow solution in a 10 mL single-dose vials. Each mL contains 2.5 mg of nicardipine hydrochloride ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 42571-394-88 - niCARdipine Hydrochloride Injection - 25 mg/10 mL (2.5 mg/mL) WARNING:MUST BE DILUTED - BEFORE INFUSION - FOR INTRAVENEOUS USE ONLY - Discard Unused ...
-
INGREDIENTS AND APPEARANCEProduct Information