Label: SOFTLIPS VANILLA- dimethicone, octinoxate, octisalate, oxybenzone ointment
- NDC Code(s): 10742-3051-1, 10742-3051-2, 10742-3051-3, 10742-3051-9
- Packager: The Mentholatum Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
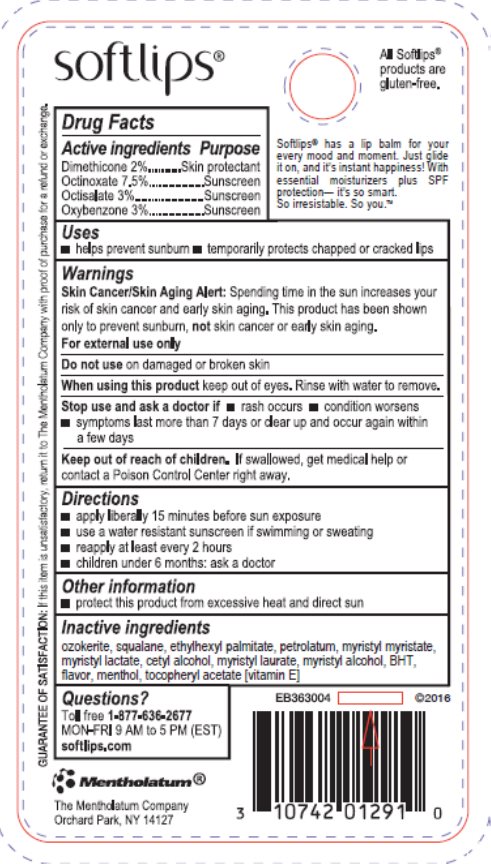
- Active ingredients
- Purpose
- Uses
-
Warnings
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to prevent sunburn, not skin cancer or early skin aging.
For external use only
- Directions
- Other information
- Inactive ingredients
- Questions?
- Principal Display Panel
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SOFTLIPS VANILLA
dimethicone, octinoxate, octisalate, oxybenzone ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10742-3051 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 20 mg in 1 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 30 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 30 mg in 1 g Inactive Ingredients Ingredient Name Strength CERESIN (UNII: Q1LS2UJO3A) SQUALANE (UNII: GW89575KF9) ETHYLHEXYL PALMITATE (UNII: 2865993309) PETROLATUM (UNII: 4T6H12BN9U) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) MYRISTYL LACTATE (UNII: 1D822OC34X) CETYL ALCOHOL (UNII: 936JST6JCN) MYRISTYL LAURATE (UNII: 58U0NZN2BT) MYRISTYL ALCOHOL (UNII: V42034O9PU) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10742-3051-1 1 in 1 BLISTER PACK 11/19/1997 1 2 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:10742-3051-9 2 in 1 BLISTER PACK 11/19/1997 2 2 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:10742-3051-2 2 g in 1 TUBE; Type 0: Not a Combination Product 11/19/1997 4 NDC:10742-3051-3 3 in 1 BLISTER PACK 03/02/2020 4 2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/19/1997 Labeler - The Mentholatum Company (002105757) Registrant - The Mentholatum Company (002105757) Establishment Name Address ID/FEI Business Operations The Mentholatum Company 002105757 manufacture(10742-3051)