Label: MAGNESIUM SULFATE IN DEXTROSE- magnesium sulfate injection
- NDC Code(s): 44567-410-24
- Packager: WG Critical Care, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MAGNESIUM SULFATE IN 5% DEXTROSE INJECTION safely and effectively. See full prescribing information for MAGNESIUM SULFATE IN 5 ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEMagnesium Sulfate in 5% Dextrose Injection, USP is indicated for: • Prevention of eclampsia in patients with preeclampsia - • Treatment of seizures and prevention of recurrent seizures in patients ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Instructions - Magnesium Sulfate in 5% Dextrose Injection is: • A clear solution. Visually inspect Magnesium Sulfate in 5% Dextrose Injection for particulate matter ...
-
3 DOSAGE FORMS AND STRENGTHSMagnesium Sulfate in 5% Dextrose Injection, USP is a clear and colorless to slightly yellow solution supplied in single-dose, single port bag: • 0.01 grams per mL (1%): ▪ 100 mL bag containing 1 ...
-
4 CONTRAINDICATIONSMagnesium Sulfate in 5% Dextrose Injection is contraindicated in patients: • with heart block or myocardial damage - • in diabetic coma - • with myasthenia gravis [see Warnings and Precautions ...
-
5 WARNINGS AND PRECAUTIONS5.1 Fetal-Neonatal Toxicity with Prolonged Use - Continuous administration of magnesium sulfate beyond 5 to 7 days in pregnant women can lead to hypocalcemia and bone abnormalities in the ...
-
6 ADVERSE REACTIONSThe following adverse reactions have been identified in clinical studies or postmarketing reports. Because these reactions are reported voluntarily from a population of uncertain size, it is not ...
-
7 DRUG INTERACTIONSTable 1 presents the potential clinical impact of medications that may be commonly administered concomitantly with Magnesium Sulfate in 5% Dextrose Injection in the clinical setting. Table 1 ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Magnesium Sulfate in 5% Dextrose Injection is indicated in pregnant women for the prevention of eclampsia in women with preeclampsia and the treatment of ...
-
10 OVERDOSAGEManifestations of magnesium toxicity include a drop in blood pressure, difficulty breathing, and disappearance of the patellar reflex. As serum magnesium rises above 4 mEq per liter, the deep ...
-
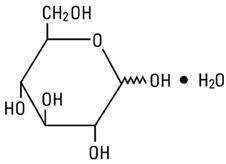
11 DESCRIPTION Magnesium Sulfate in 5% Dextrose Injection, USP is a sterile, nonpyrogenic solution of magnesium sulfate heptahydrate and dextrose in water for injection for intravenous use. Each 100 mL contains ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Magnesium prevents seizures in patients with preeclampsia and controls seizures in patients with eclampsia by blocking neuromuscular transmission and decreasing the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Studies with Magnesium Sulfate in 5% Dextrose Injection have not been performed to evaluate carcinogenic potential, mutagenic potential ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING Magnesium Sulfate in 5% Dextrose Injection, USP is supplied in a single-dose, single port bag with an aluminum overwrap. The infusion bags and ports are not made with natural rubber ...
-
17 PATIENT COUNSELING INFORMATION Magnesium Sulfate in 5% Dextrose Injection is typically administered to pregnancy women in emergent situations. When feasible, advise the patient and family of the following: Fetal-Neonatal ...
-
SPL UNCLASSIFIED SECTIONManufactured for: WG Critical Care, LLC - Paramus, NJ 07652 - Made in Switzerland
-
PRINCIPAL DISPLAY PANEL - 100 mL Bag OverwrapNDC 44567-410-24 100 mL - Magnesium Sulfate - in 5% Dextrose Injection, USP - (0.081 mEq Mg++/mL) 10 mg/mL - 1 g Total
-
INGREDIENTS AND APPEARANCEProduct Information