Label: PEMETREXED injection, powder, lyophilized, for solution
- NDC Code(s): 0409-1060-01, 0409-1061-01, 0409-1062-01
- Packager: Hospira, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated July 22, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PEMETREXED FOR INJECTION safely and effectively. See full prescribing information for PEMETREXED FOR INJECTION. PEMETREXED FOR ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Non-Squamous Non-Small Cell Lung Cancer (NSCLC) Pemetrexed for Injection is indicated: In combination with cisplatin for the initial treatment of patients with locally advanced or ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage for Non-Squamous NSCLC - The recommended dose of Pemetrexed for Injection when administered with cisplatin for initial treatment of locally advanced or metastatic ...
-
3 DOSAGE FORMS AND STRENGTHSFor injection: 100 mg, 500 mg or 1 gram pemetrexed as a white to light-yellow or green-yellow lyophilized powder in single-dose vials for reconstitution.
-
4 CONTRAINDICATIONSPemetrexed for Injection is contraindicated in patients with a history of severe hypersensitivity reaction to pemetrexed [see Adverse Reactions (6.1)].
-
5 WARNINGS AND PRECAUTIONS5.1 Myelosuppression and Increased Risk of Myelosuppression without Vitamin Supplementation - Pemetrexed for Injection can cause severe myelosuppression resulting in a requirement for ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in other sections of the labeling: Myelosuppression [see Warnings and Precautions (5.1)] Renal failure [see Warnings and ...
-
7 DRUG INTERACTIONSEffects of Ibuprofen on Pemetrexed - Ibuprofen increases exposure (AUC) of pemetrexed [see Clinical Pharmacology (12.3)]. In patients with creatinine clearance between 45 mL/min and 79 ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings from animal studies and its mechanism of action, Pemetrexed for Injection can cause fetal harm when administered to a pregnant woman [see ...
-
10 OVERDOSAGENo drugs are approved for the treatment of Pemetrexed for Injection overdose. Based on animal studies, administration of leucovorin may mitigate the toxicities of Pemetrexed for Injection ...
-
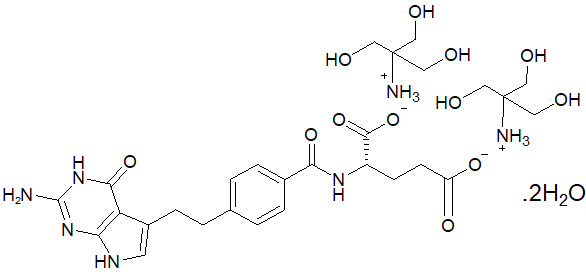
11 DESCRIPTIONPemetrexed is a folate analog metabolic inhibitor. The drug substance, pemetrexed ditromethamine, has the chemical name L-glutamic acid, N-[4-[2-(2-amino-4,7-dihydro-4-oxo-1H-pyrrolo[2,3-d ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Pemetrexed for Injection is a folate analog metabolic inhibitor that disrupts folate-dependent metabolic processes essential for cell replication. In vitro studies show ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No carcinogenicity studies have been conducted with pemetrexed. Pemetrexed was clastogenic in an in vivo micronucleus assay in mouse ...
-
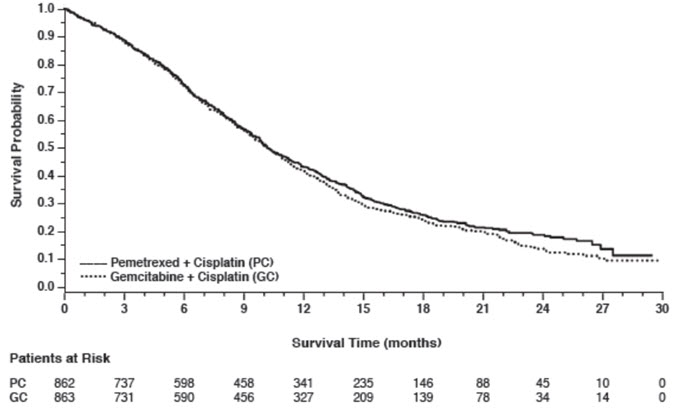
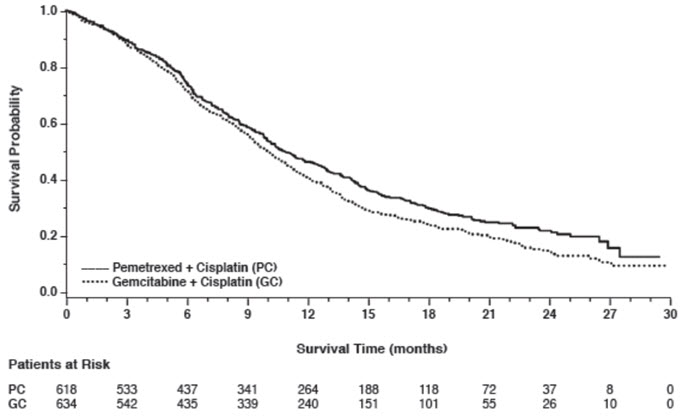
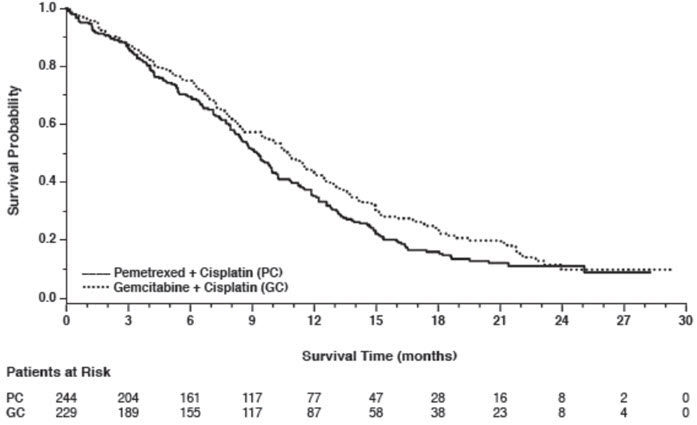
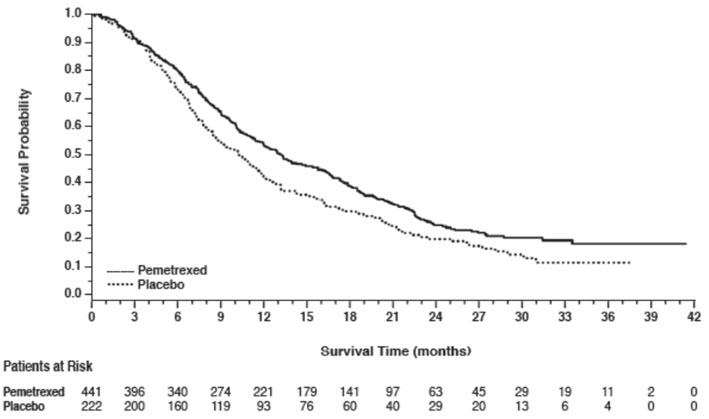
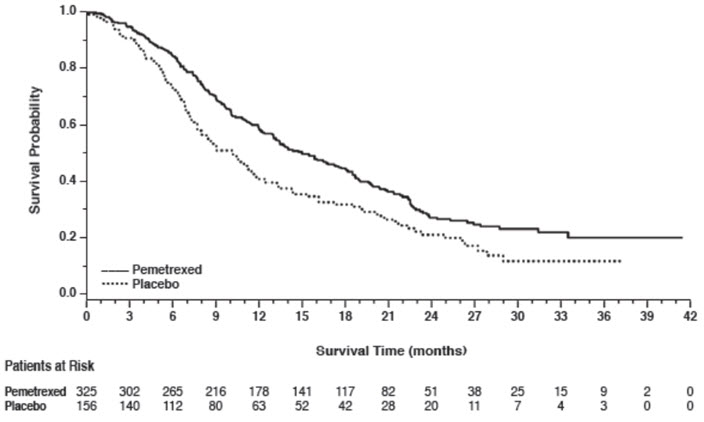
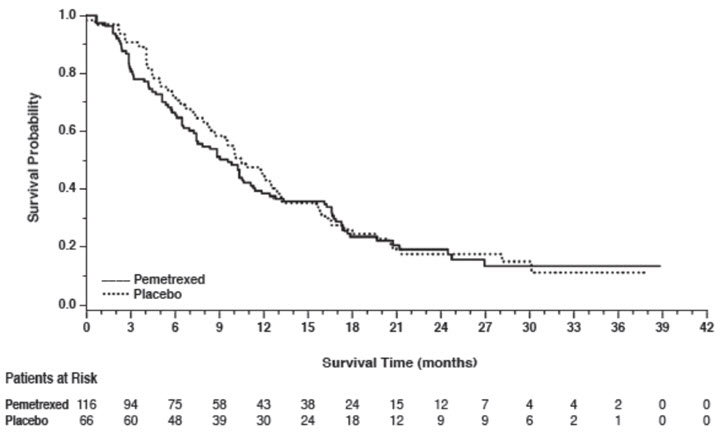
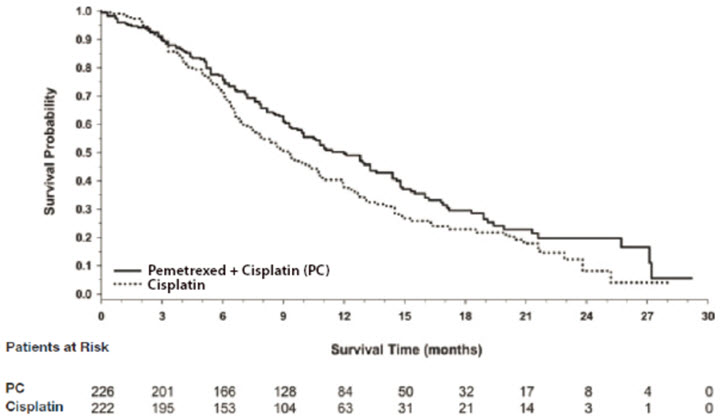
14 CLINICAL STUDIES14.1 Non-Squamous NSCLC - Initial Treatment in Combination with Cisplatin - The efficacy of pemetrexed was evaluated in Study JMDB (NCT00087711), a multi-center, randomized (1:1), open-label ...
-
15 REFERENCESOSHA Hazardous Drugs. OSHA. [http://www.osha.gov/hazardous-drugs]
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - Pemetrexed for Injection is a white-to-light yellow or green-yellow lyophilized powder supplied in single-dose vials for reconstitution for intravenous infusion. Unit of ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Premedication and Concomitant Medication: Instruct patients to take folic acid as directed and to keep ...
-
SPL UNCLASSIFIED SECTIONThis product's labeling may have been updated. For most recent prescribing information, please visit www.pfizer.com. Manufactured by: Zydus Hospira Oncology Private Ltd. Ahmedabad 382-213, Gujarat ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - PEMETREXED (peh-meh-TREX-ed) FOR INJECTION - for intravenous use - This Patient Information has been approved by the U.S. Food and Drug Administration.Issued: June ...
-
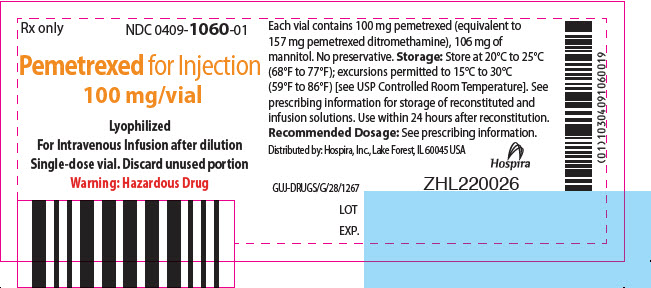
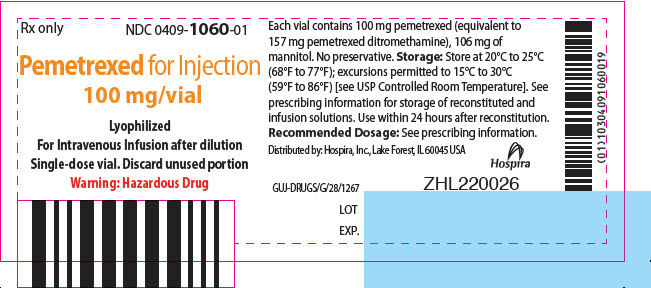
PRINCIPAL DISPLAY PANEL - 100 mg Vial LabelRx only - NDC 0409-1060-01 - Pemetrexed for Injection - 100 mg/vial - Lyophilized - For Intravenous Infusion after dilution - Single-dose vial. Discard unused portion - Warning: Hazardous Drug
-
PRINCIPAL DISPLAY PANEL - 100 mg Vial Carton1 x 100 mg/vial - NDC 0409-1060-01 - Rx only - Pemetrexed - for Injection - 100 mg/vial - Lyophilized - For Intravenous Infusion - after dilution - Single-dose vial. Discard unused portion. Hospira - Warning ...
-
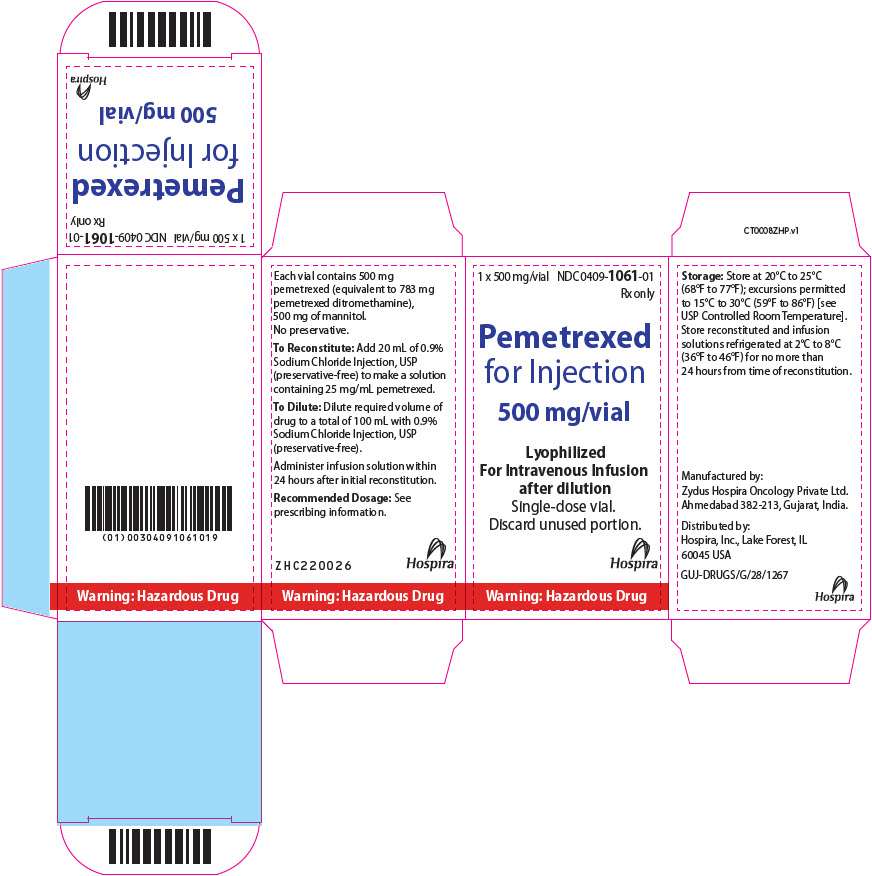
PRINCIPAL DISPLAY PANEL - 500 mg Vial LabelRx only - NDC 0409-1061-01 - Pemetrexed for Injection - 500 mg/vial - Lyophilized - For Intravenous Infusion after dilution - Single-dose vial. Discard unused portion - Warning: Hazardous Drug
-
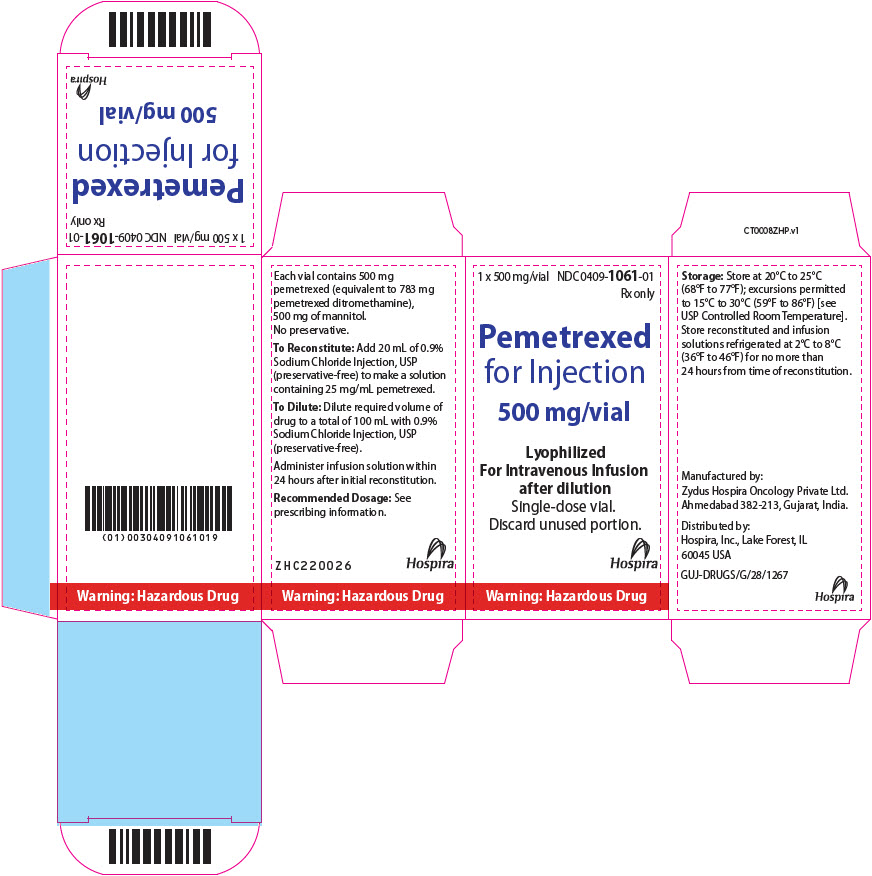
PRINCIPAL DISPLAY PANEL - 500 mg Vial Carton1 x 500 mg/vial - NDC 0409-1061-01 - Rx only - Pemetrexed - for Injection - 500 mg/vial - Lyophilized - For Intravenous Infusion - after dilution - Single-dose vial. Discard unused portion. Hospira - Warning ...
-
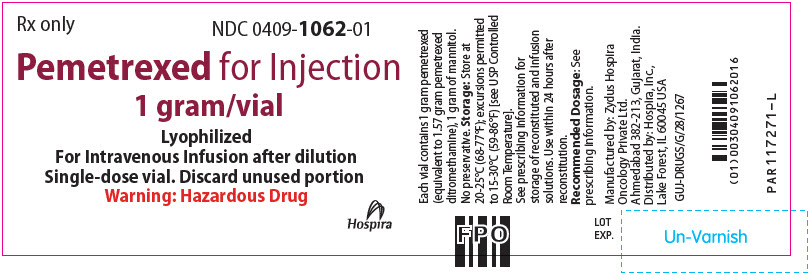
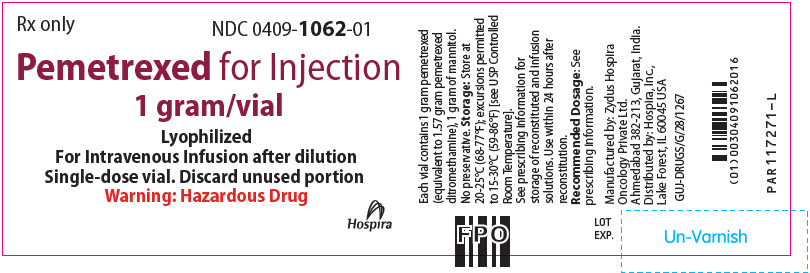
PRINCIPAL DISPLAY PANEL - 1 gram Vial LabelRx only - NDC 0409-1062-01 - Pemetrexed for Injection - 1 gram/vial - Lyophilized - For Intravenous Infusion after dilution - Single-dose vial. Discard unused portion - Warning: Hazardous Drug - Hospira
-
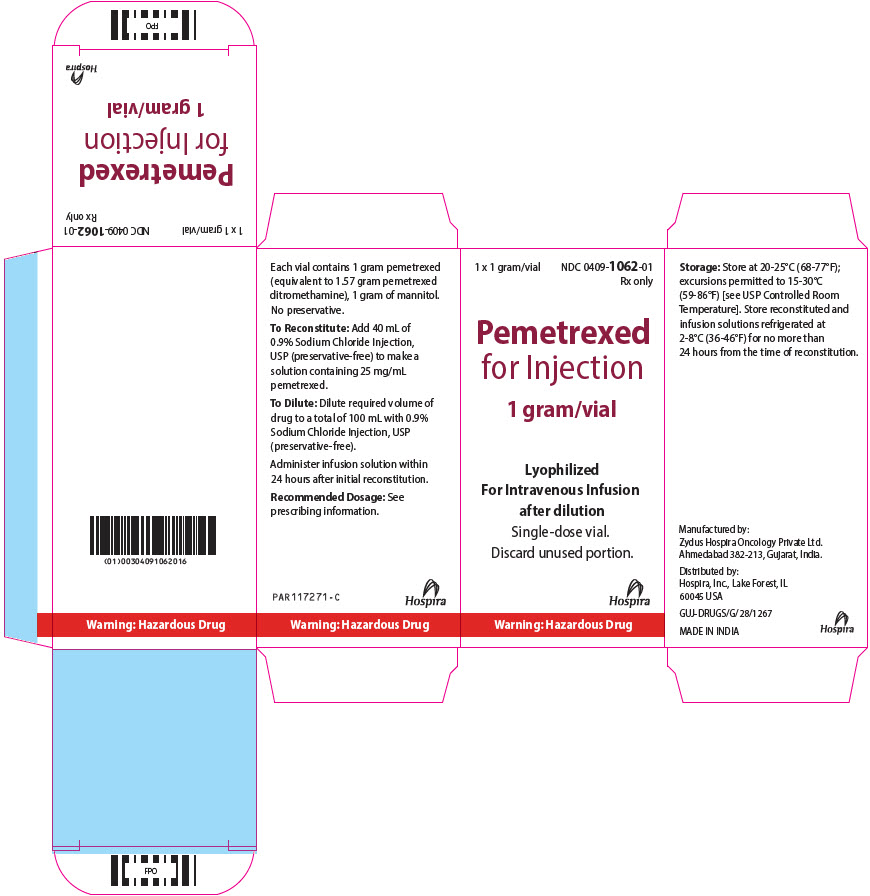
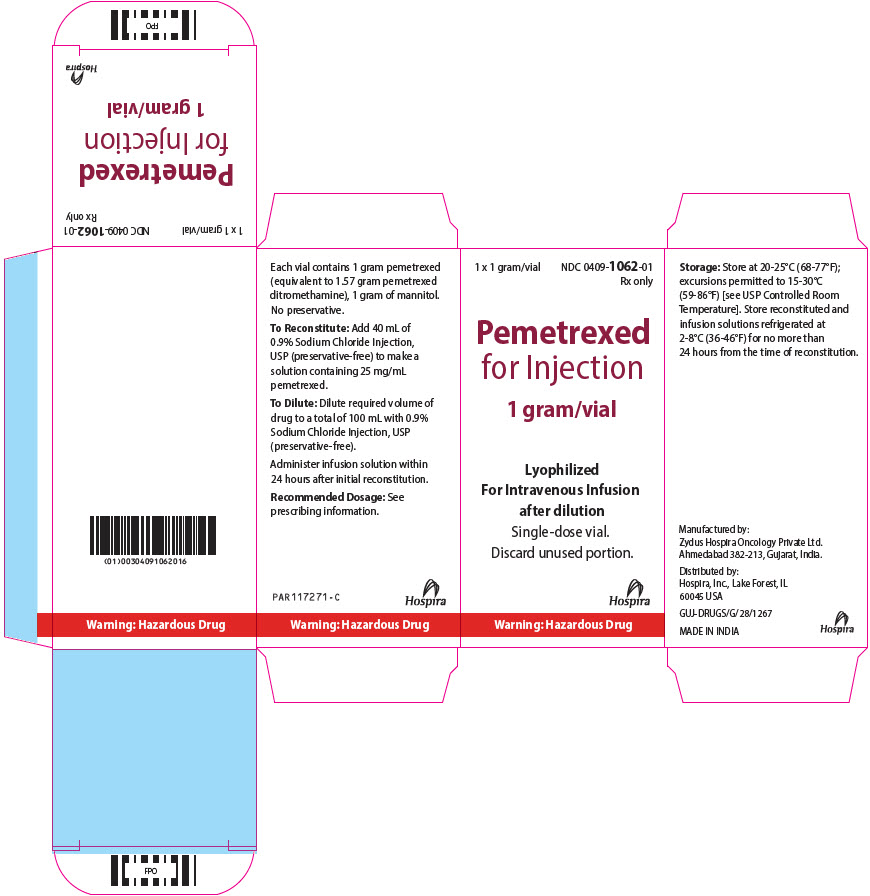
PRINCIPAL DISPLAY PANEL - 1 gram Vial Carton1 x 1 gram/vial - NDC 0409-1062-01 - Rx only - Pemetrexed - for Injection - 1 gram/vial - Lyophilized - For Intravenous Infusion - after dilution - Single-dose vial. Discard unused portion. Hospira - Warning ...
-
INGREDIENTS AND APPEARANCEProduct Information