Label: PROPRANOLOL HYDROCHLORIDE tablet
- NDC Code(s): 51407-235-01, 51407-235-10, 51407-236-01, 51407-236-10, view more
- Packager: Golden State Medical Supply, Inc.
- This is a repackaged label.
- Source NDC Code(s): 0378-0182, 0378-0183, 0378-0184, 0378-0185, view more
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 3, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
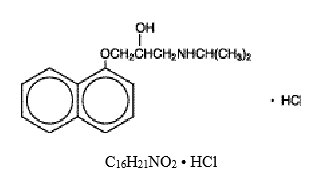
Propranolol hydrochloride is a synthetic beta-adrenergic receptor blocking agent chemically described as (±)-1-(Isopropylamino)-3-(1-naphthyloxy)-2-propanolol hydrochloride. Its molecular and ...
-
CLINICAL PHARMACOLOGYGeneral - Propranolol is a nonselective beta-adrenergic receptor blocking agent possessing no other autonomic nervous system activity. It specifically competes with beta-adrenergic receptor ...
-
INDICATIONS AND USAGEHypertension - Propranolol hydrochloride tablets are indicated in the management of hypertension. It may be used alone or used in combination with other antihypertensive agents, particularly a ...
-
CONTRAINDICATIONSPropranolol is contraindicated in 1) cardiogenic shock; 2) sinus bradycardia and greater than first degree block; 3) bronchial asthma; and 4) in patients with known hypersensitivity to propranolol ...
-
WARNINGSAngina Pectoris - There have been reports of exacerbation of angina and, in some cases, myocardial infarction, following abrupt discontinuance of propranolol therapy. Therefore, when ...
-
PRECAUTIONSGeneral - Propranolol should be used with caution in patients with impaired hepatic or renal function. Propranolol hydrochloride tablets are not indicated for the treatment of hypertensive ...
-
ADVERSE REACTIONSThe following adverse events were observed and have been reported in patients using propranolol. Cardiovascular:Bradycardia; congestive heart failure; intensification of AV block; hypotension ...
-
OVERDOSAGEPropranolol is not significantly dialyzable. In the event of overdosage or exaggerated response, the following measures should be employed: General - If ingestion is or may have been recent ...
-
DOSAGE AND ADMINISTRATIONGeneral - Because of the variable bioavailability of propranolol, the dose should be individualized based on response. Hypertension - The usual initial dosage is 40 mg propranolol hydrochloride ...
-
HOW SUPPLIEDPropranolol Hydrochloride Tablets, USP are available containing 10 mg, 20 mg, 40 mg, 60 mg or 80 mg of propranolol hydrochloride, USP. The 10 mg tablets are orange, round, scored tablets debossed ...
-
PRINCIPAL DISPLAY PANEL – 10 mgNDC 51407-235-01 - Propranolol - Hydrochloride - Tablets, USP - 10 mg - Rx only 100 Tablets - Dispense in a tight, light-resistant container as defined in the USP - using a ...
-
PRINCIPAL DISPLAY PANEL – 20 mgNDC 51407-236-01 - Propranolol - Hydrochloride - Tablets, USP - 20 mg - Rx only 100 Tablets - Dispense in a tight, light-resistant container as defined in the USP - using a ...
-
PRINCIPAL DISPLAY PANEL – 40 mgNDC 51407-237-01 - Propranolol - Hydrochloride - Tablets, USP - 40 mg - Rx only 100 Tablets - Dispense in a tight, light-resistant container as defined in the USP - using a ...
-
PRINCIPAL DISPLAY PANEL – 60 mgNDC 51407-239-01 - Propranolol - Hydrochloride - Tablets, USP - 60 mg - Rx only 100 Tablets - Dispense in a tight, light-resistant container as defined in the USP - using a ...
-
PRINCIPAL DISPLAY PANEL – 80 mgNDC 51407-238-01 - Propranolol - Hydrochloride - Tablets, USP - 80 mg - Rx only 100 Tablets - Dispense in a tight, light-resistant container as - defined in the USP using a ...
-
INGREDIENTS AND APPEARANCEProduct Information