Label: NELARABINE injection
- NDC Code(s): 70710-1839-1, 70710-1839-8
- Packager: Zydus Pharmaceuticals USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 16, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NELARABINE INJECTION safely and effectively. See full prescribing information for NELARABINE INJECTION. NELARABINE injection, for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: NEUROLOGIC ADVERSE REACTIONS
Severe neurologic adverse reactions have been reported with the use of nelarabine. These adverse reactions have included altered mental states including severe somnolence, central nervous system effects including convulsions, and peripheral neuropathy ranging from numbness and paresthesias to motor weakness and paralysis. There have also been reports of adverse reactions associated with demyelination, and ascending peripheral neuropathies similar in appearance to Guillain-Barr¡SR syndrome [see Warnings and Precautions (5.1)].
Full recovery from these adverse reactions has not always occurred with cessation of therapy with nelarabine. Monitor frequently for signs and symptoms of neurologic toxicity during treatment with nelarabine. Discontinue nelarabine for neurologic adverse reactions of NCI Common Toxicity Criteria for Adverse Events (CTCAE) Grade 2 or greater [see Warnings and Precautions (5.1)].
Close -
1 INDICATIONS AND USAGENelarabine Injection is indicated for the treatment of T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma (T-LBL) in adult and pediatric patients age 1 year and older ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - This product is for intravenous use only. Adult Dosage: The recommended adult dose of nelarabine injection is 1,500 mg/m2 administered by intravenous infusion over 2 ...
-
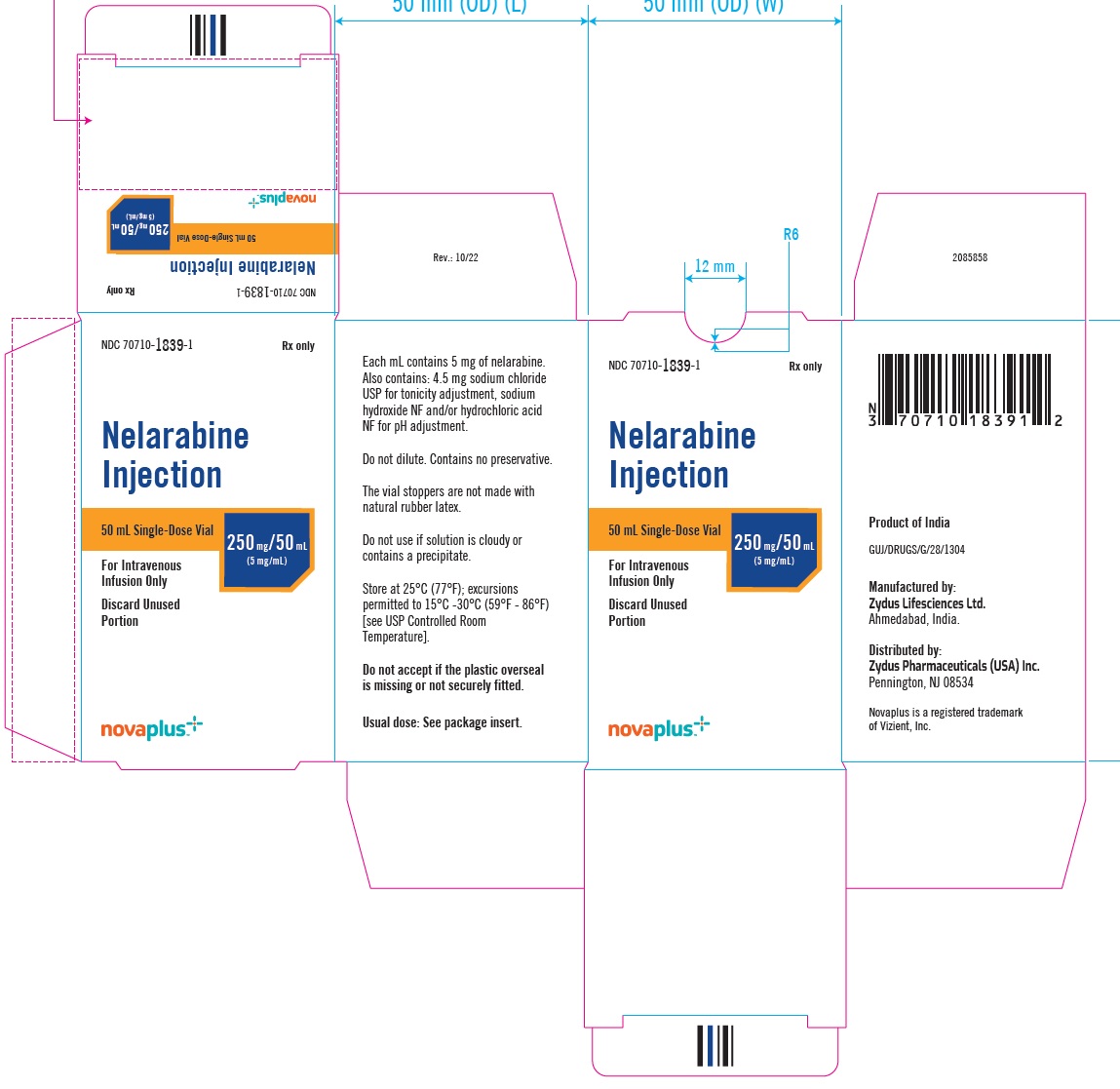
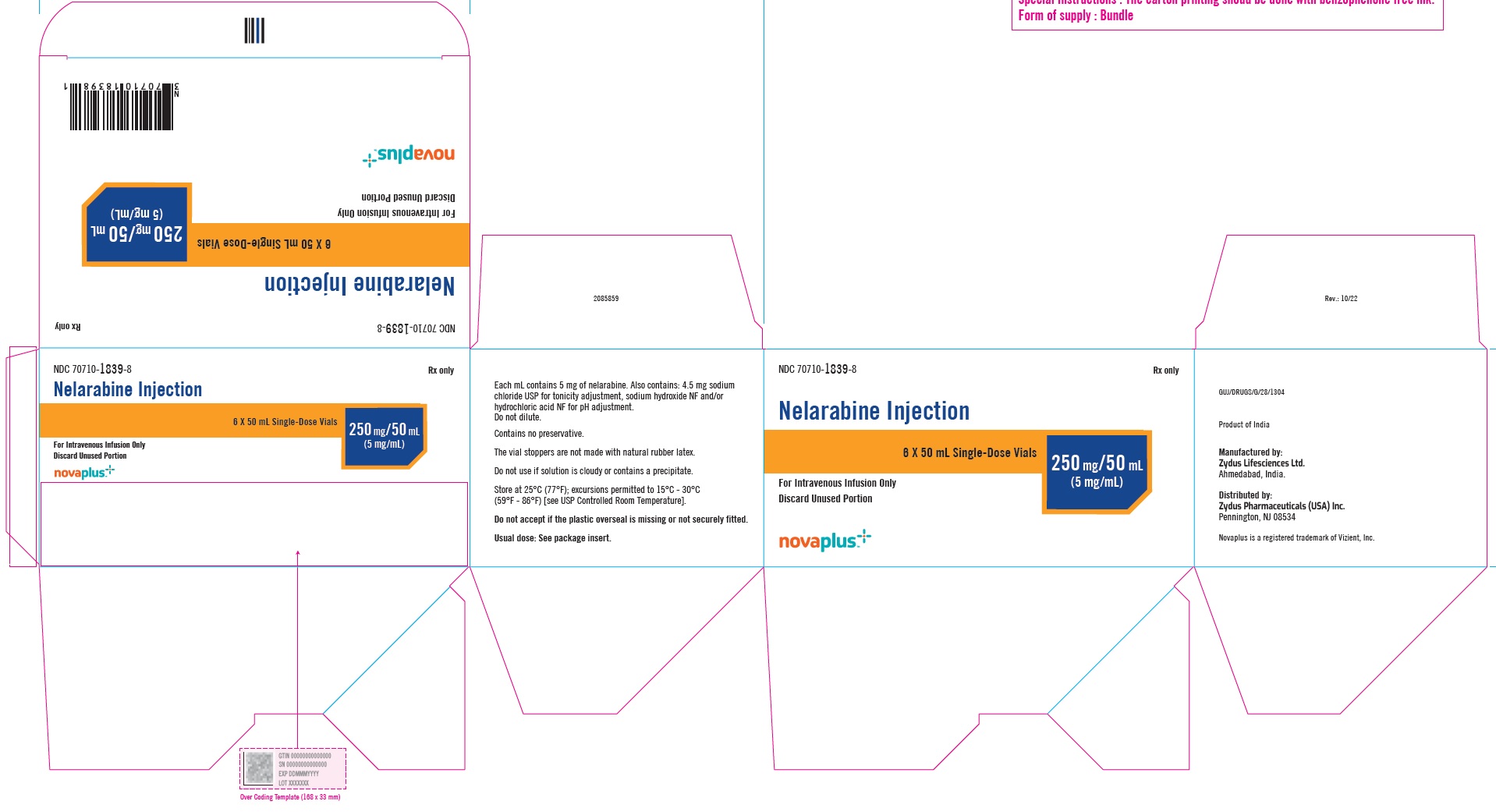
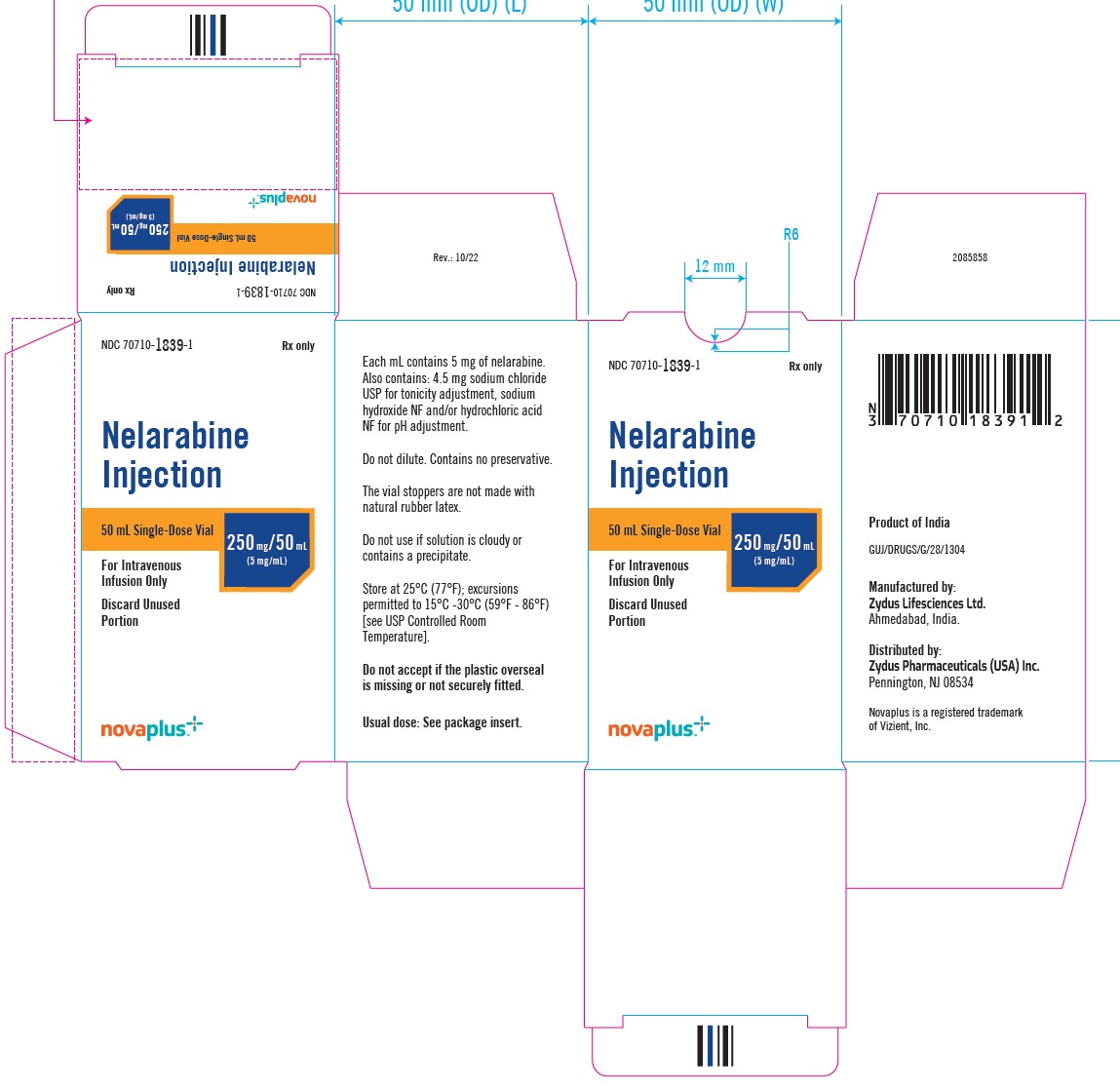
3 DOSAGE FORMS AND STRENGTHSNelarabine Injection: 250 mg/50 mL (5 mg/mL) is supplied as a clear, colorless, sterile solution in Type I, clear glass single-dose vials with a grey bromobutyl rubber stopper (not made with ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Neurologic Adverse Reactions - Nervous system adverse reactions of any grade were reported for 223 (76%) adult patients across the Phase I and Phase II trials, and Grade 3 or higher (severe ...
-
6 ADVERSE REACTIONSThe following clinically-significant adverse reactions are discussed in greater detail in other sections of the label: Neurologic [see Boxed Warning, Warnings and Precautions (5.1)] Hematologic ...
-
7 DRUG INTERACTIONSAdministration of nelarabine in combination with adenosine deaminase (ADA) inhibitors, such as pentostatin, is not recommended [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on its mechanism of action and findings in animal studies, nelarabine can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology ...
-
10 OVERDOSAGEThere is no known antidote for overdoses of nelarabine. It is anticipated that overdosage would result in severe neurotoxicity (possibly including paralysis, coma), myelosuppression, and ...
-
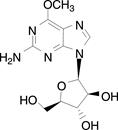
11 DESCRIPTIONNelarabine is a prodrug of the cytotoxic deoxyguanosine analogue, 9-β-D arabinofuranosylguanine (ara-G). The chemical name for nelarabine is 2-amino-9-β-D-arabinofuranosyl-6-methoxy-9H-purine ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Nelarabine is a prodrug of the deoxyguanosine analogue 9-β-D-arabinofuranosylguanine (ara-G), a nucleoside metabolic inhibitor. Nelarabine is demethylated by ADA to ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity testing of nelarabine has not been done. However, nelarabine was mutagenic when tested in vitro in L5178Y/TK mouse ...
-
14 CLINICAL STUDIES14.1 Adult Clinical Trial in Relapsed or Refractory T-ALL and T-LBL - The safety and efficacy of nelarabine in adult patients were studied in a clinical trial which included 39 treated patients ...
-
15 REFERENCES1. "OSHA Hazardous Drugs." OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html
-
16 HOW SUPPLIED/STORAGE AND HANDLINGNelarabine Injection is supplied as a clear, colorless, sterile solution in Type I, clear glass single-dose vials with a grey bromobutyl rubber stopper (not made with natural rubber latex) and an ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Hematologic Adverse Reactions - Advise patients that leukopenia, thrombocytopenia, anemia, and neutropenia ...
-
SPL UNCLASSIFIED SECTIONManufactured by - Zydus Lifesciences Ltd. Ahmedabad, India - Distributed by - Zydus Pharmaceuticals (USA) Inc. Pennington, NJ 08534 - Novaplus is a registered trademark of Vizient, Inc. Rev. ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - Nelarabine (nel ar' a been ) Injection - Read the Patient Information that comes with nelarabine injection before you or your child starts treatment with nelarabine ...
-
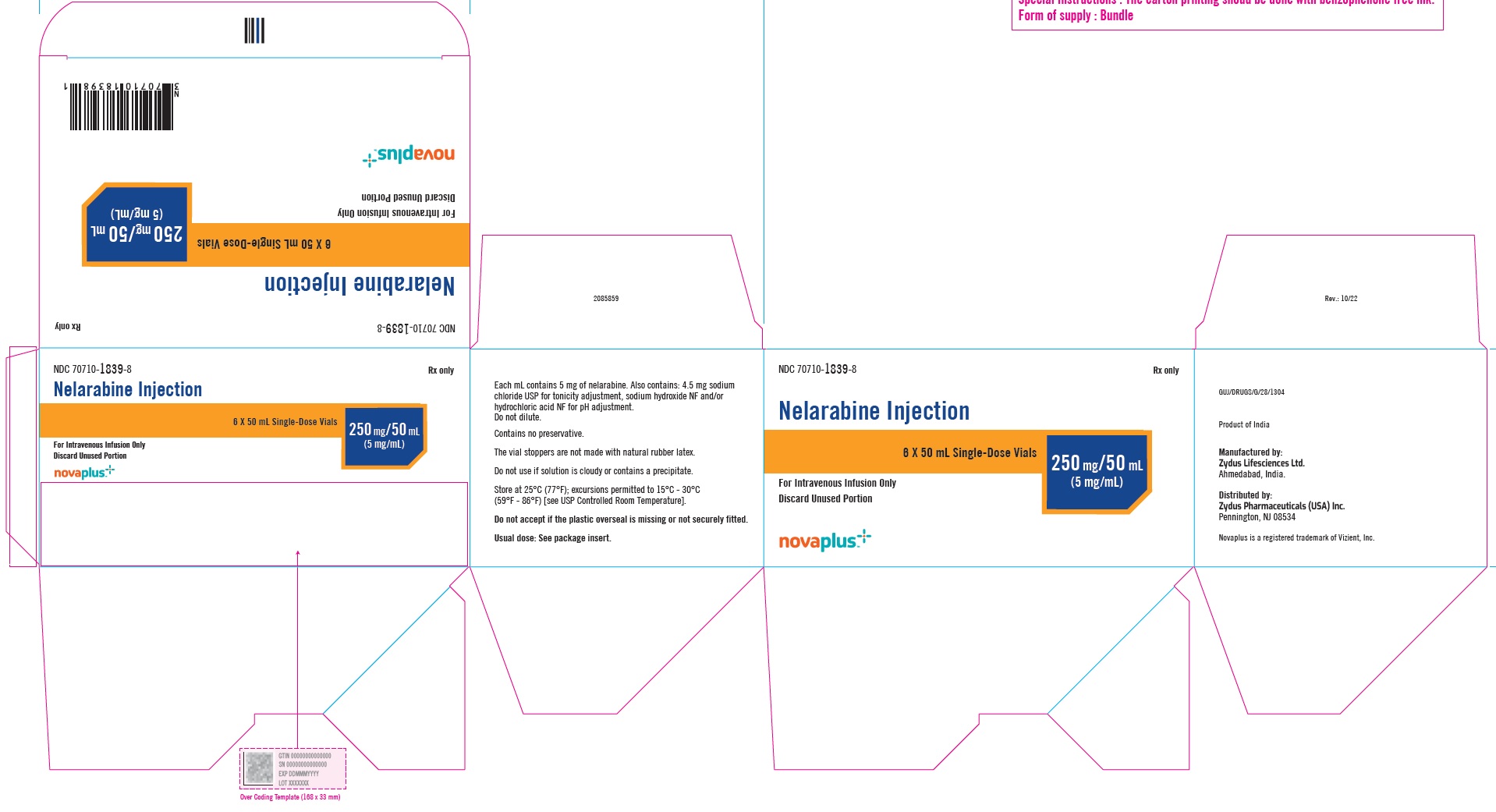
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 70710-1839-1 - Nelarabine Injection 250 mg/5 mL (5 mg/mL) 50 mL Single-Dose Vial - For Intravenous Infusion Only - Rx Only - NDC 70710-1839-1 - Nelarabine Injection 250 mg/5 mL (5 mg/mL) 50 mL ...
-
INGREDIENTS AND APPEARANCEProduct Information