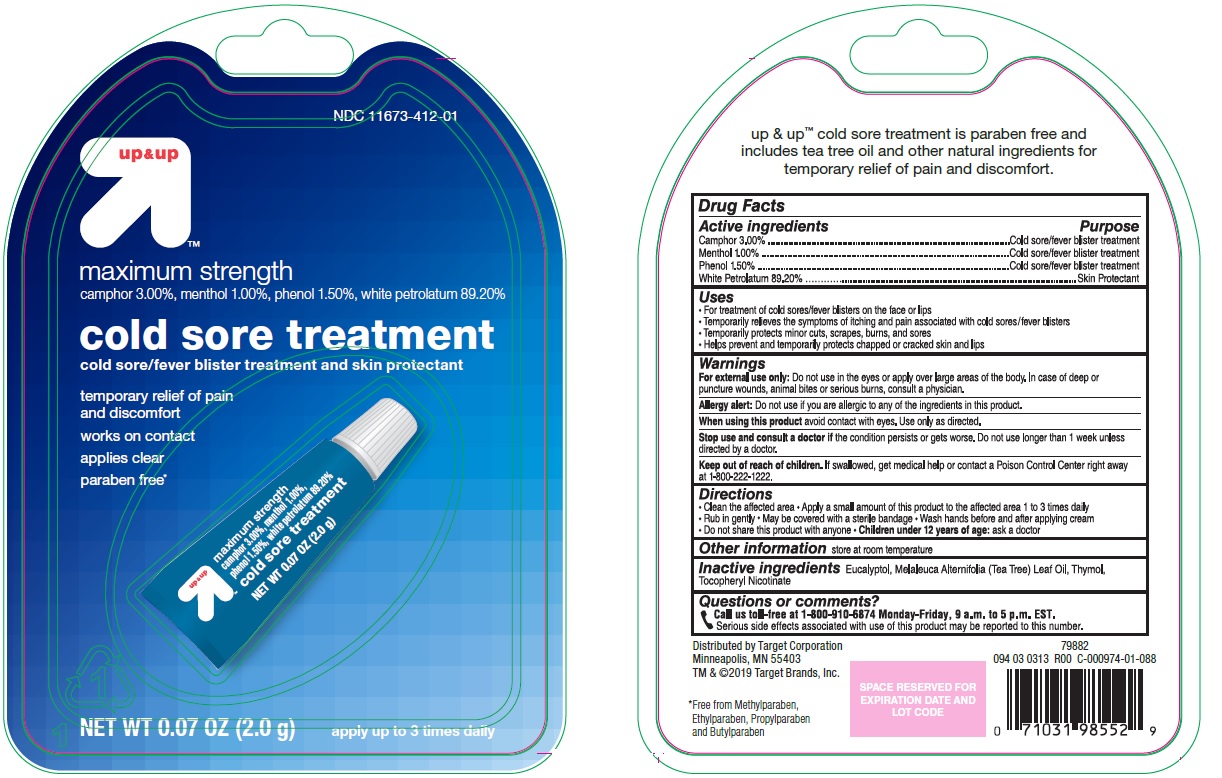
Label: MAXIMUM STRENGTH COLD SORE TREATMENT- camphor, menthol, phenol, white petrolatum gel
- NDC Code(s): 11673-412-01
- Packager: Target Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
-
Warnings
For external use only: Do not use in the eyes or apply over large areas of the body. In case of deep or puncture wounds, animal bites or serious burns, consult a physician.
Allergy alert: Do not use if you are allergic to any of the ingredients in this product.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- Principle Display Panel
-
INGREDIENTS AND APPEARANCE
MAXIMUM STRENGTH COLD SORE TREATMENT
camphor, menthol, phenol, white petrolatum gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11673-412 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 30 mg in 1 g MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 10 mg in 1 g PHENOL (UNII: 339NCG44TV) (PHENOL - UNII:339NCG44TV) PHENOL 15 mg in 1 g PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 892 mg in 1 g Inactive Ingredients Ingredient Name Strength THYMOL (UNII: 3J50XA376E) EUCALYPTOL (UNII: RV6J6604TK) MELALEUCA ALTERNIFOLIA LEAF (UNII: G43C57162K) TOCOPHERYL NICOTINATE (UNII: WI1J5UCY5C) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11673-412-01 2 g in 1 TUBE; Type 0: Not a Combination Product 06/27/2019 02/23/2026 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 06/27/2019 02/23/2026 Labeler - Target Corporation (006961700)