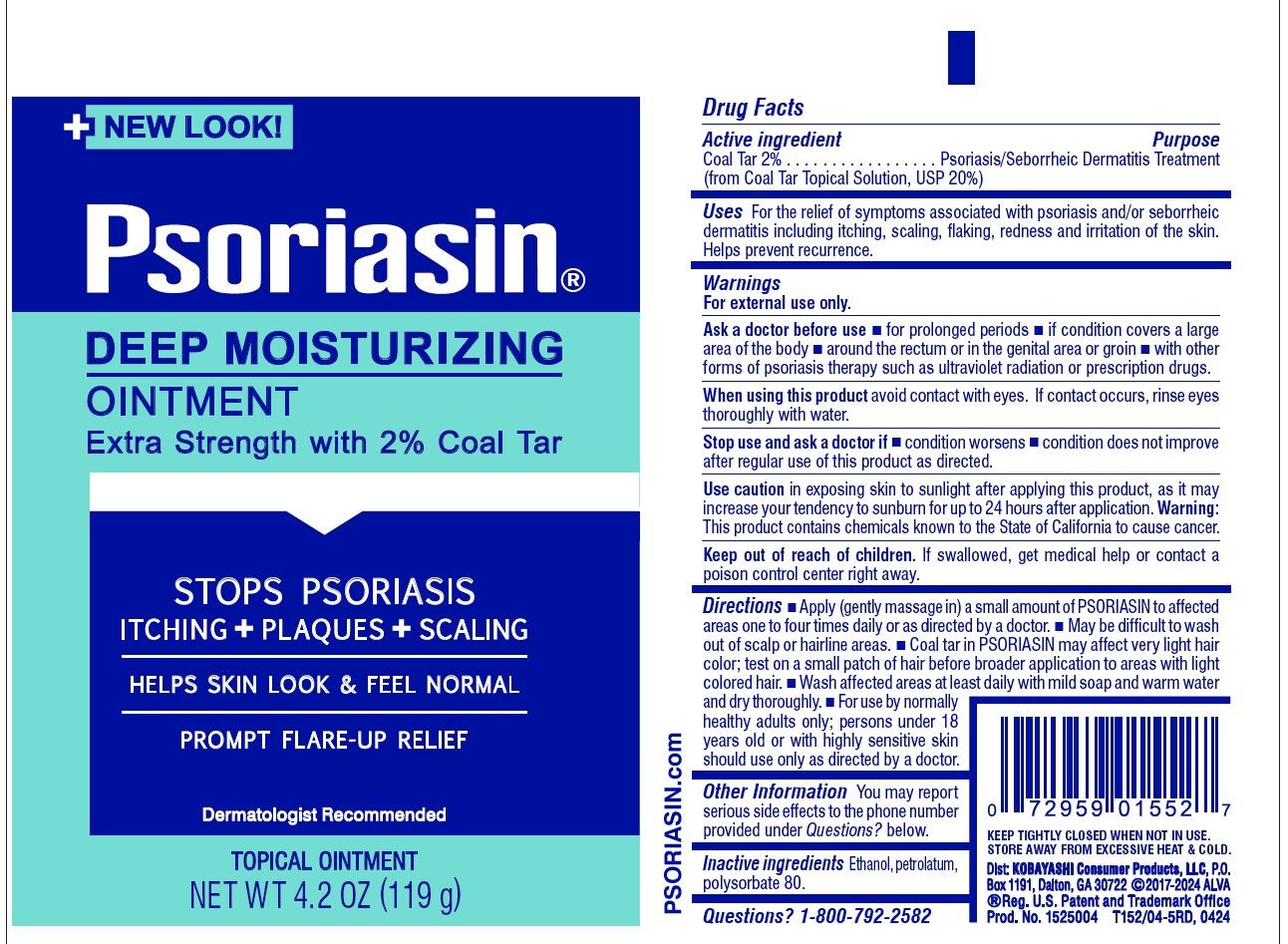
Label: PSORIASIN DEEP MOISTURIZING- coal tar ointment
- NDC Code(s): 52389-752-02, 52389-752-42
- Packager: Kobayashi Healthcare International, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Ask a doctor before use
- WHEN USING
-
Stop use and ask a doctor if
- condition worsens
- condition does not improve after regular use of this product as directed.
Use caution in exposing skin to sunlight after applying this product, as it may increase your tendency to sunburn for up to 24 hours after application.
Warning: This product contains chemicals known to the State of California to cause cancer.
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- Apply (gently massage in) a small amount of Psoriasin to affected areas one to four times daily or as directed by a doctor.
- May be difficult to wash out of scalp or hairline areas.
- Coal tar in Psoriasin may affect very light hair color; test first on a small patch of hair before broader application to areas with light colored hair.
- Wash affected areas at least daily with mild soap and warm water and dry thoroughly.
- For use by normally healthy adults only; persons under 18 years old or with highly sensitive or allergic skin should use only as directed by a doctor.
- Other information
- Inactive ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PSORIASIN DEEP MOISTURIZING
coal tar ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52389-752 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COAL TAR (UNII: R533ESO2EC) (COAL TAR - UNII:R533ESO2EC) COAL TAR 20 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) ALCOHOL (UNII: 3K9958V90M) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52389-752-42 1 in 1 CARTON 06/21/2019 1 119 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:52389-752-02 119 g in 1 TUBE; Type 0: Not a Combination Product 06/21/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 06/21/2019 Labeler - Kobayashi Healthcare International, Inc. (156391729) Registrant - Kobayashi America Manufacturing, LLC (079852150)