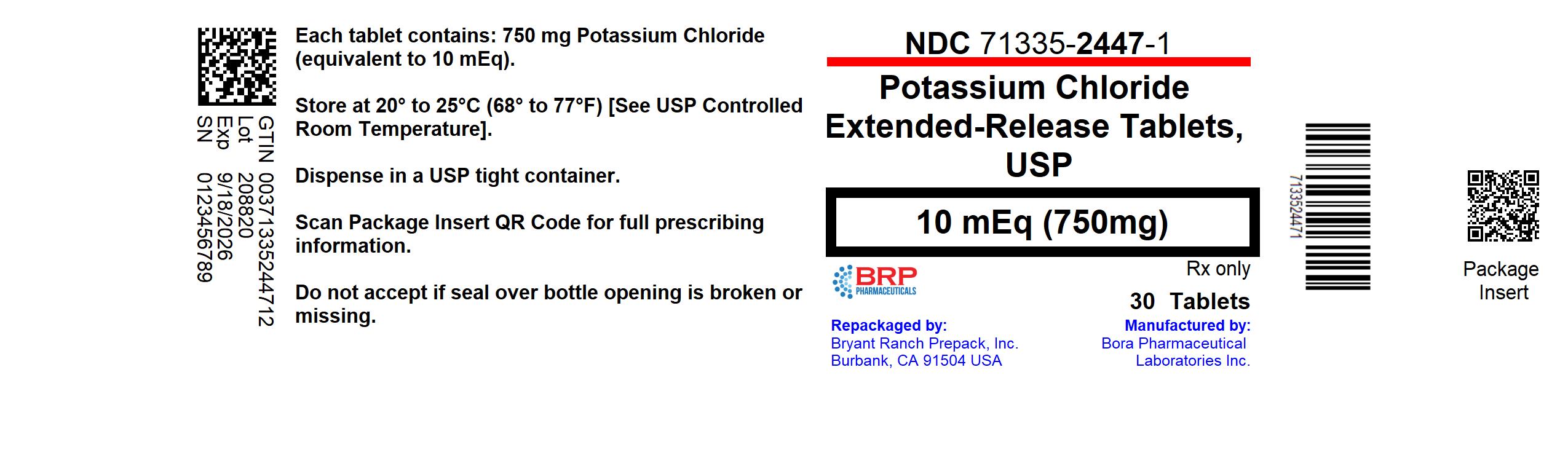
Label: POTASSIUM CHLORIDE EXTENDED-RELEASE- potassium chloride tablet, extended release
- NDC Code(s): 71335-2447-1, 71335-2447-2, 71335-2447-3, 71335-2447-4, view more
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 24979-231
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use POTASSIUM CHLORIDE EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for POTASSIUM CHLORIDE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEPotassium chloride extended-release tablets are indicated for the treatment and prophylaxis of hypokalemia with or without metabolic alkalosis, in patients for whom dietary management with ...
-
2 DOSAGE AND ADMINISTRATION2.1 Monitoring and Administration - If serum potassium concentration is less than 2.5 mEq/L, use intravenous potassium instead of oral supplementation. Monitoring - Monitor serum potassium and ...
-
3 DOSAGE FORMS AND STRENGTHSPotassium Chloride Extended-release Tablets, USP, 10 mEq (750 mg) are yellow film coated capsule shaped tablets; plain on one side and debossed with "P10" on the other side. Potassium Chloride ...
-
4 CONTRAINDICATIONSPotassium chloride is contraindicated in patients on triamterene or amiloride.
-
5 WARNINGS AND PRECAUTIONS5.1 Gastrointestinal Adverse Reactions - Solid oral dosage forms of potassium chloride can produce ulcerative and/or stenotic lesions of the gastrointestinal tract, particularly when the drug ...
-
6 ADVERSE REACTIONSThe following adverse reactions have been identified with use of oral potassium salts. Because these reactions are reported voluntarily from a population of uncertain size, it is not always ...
-
7 DRUG INTERACTIONS7.1 Triamterene and Amiloride - Use with triamterene or amiloride can produce severe hyperkalemia. Avoid concomitant use [see Contraindications (4)]. 7.2 Renin-Angiotensin-Aldosterone System ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no human data related to use of potassium chloride extended-release tablets during pregnancy, and animal reproduction studies have not been conducted ...
-
10 OVERDOSAGE10.1 Symptoms - The administration of oral potassium salts to persons with normal excretory mechanisms for potassium rarely causes serious hyperkalemia. However, if excretory mechanisms are ...
-
11 DESCRIPTIONPotassium chloride extended-release tablets are solid oral dosage form of potassium chloride containing 750 mg and 1500 mg of potassium chloride, USP, equivalent to 10 mEq and 20 mEq of potassium ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The potassium ion (K+) is the principal intracellular cation of most body tissues. Potassium ions participate in a number of essential physiological processes including ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity, mutagenicity and fertility studies in animals have not been performed. Potassium is a normal dietary ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGPotassium chloride extended-release tablets, USP contain 750 mg (equivalent to 10 mEq of potassium, respectively). Potassium chloride extended-release tablets are provided as extended-release ...
-
17 PATIENT COUNSELING INFORMATIONInform patients to take each dose with meals and with a full glass of water or other liquid, and to not crush, chew, or suck the tablets. Advise patients to seek medical attention if tarry stools ...
-
SPL UNCLASSIFIED SECTIONManufactured for: TWi Pharmaceuticals USA, Inc. Paramus, NJ 07652 - Manufactured by: Bora Pharmaceutical Laboratories Inc. Jhunan, Taiwan - Revised 01/2023 - LA-3022-05
-
PRINCIPAL DISPLAY PANELPotassium Chloride ER 10 mEq K Tablet
-
INGREDIENTS AND APPEARANCEProduct Information