Label: MIRTAZAPINE tablet, film coated
- NDC Code(s): 66993-606-04, 66993-606-30, 66993-607-04, 66993-607-30, view more
- Packager: Prasco Laboratories
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MIRTAZAPINE TABLETS safely and effectively. See full prescribing information for MIRTAZAPINE TABLETS. MIRTAZAPINE tablets, for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric and young adult patients in short-term studies. Closely monitor all antidepressant-treated patients for clinical worsening, and for emergence of suicidal thoughts and behaviors [see Warnings and Precautions (5.1)]. Mirtazapine tablets are not approved for use in pediatric patients [see Use in Specific Populations (8.4)].
Close -
1 INDICATIONS AND USAGEMirtazapine tablets are indicated for the treatment of major depressive disorder (MDD) in adults [see Clinical Studies (14)].
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended starting dose of mirtazapine tablets is 15 mg once daily, administered orally, preferably in the evening prior to sleep. If patients do not have an ...
-
3 DOSAGE FORMS AND STRENGTHSMirtazapine tablets are supplied as: 7.5 mg tablets: Orange, oval, film-coated tablet debossed with “M1” on one side and plain on the other side. 15 mg tablets: Yellow, oblong, biconvex ...
-
4 CONTRAINDICATIONSMirtazapine tablets are contraindicated in patients: Taking, or within 14 days of stopping, MAOIs (including the MAOIs linezolid and intravenous methylene blue) because of an increased risk of ...
-
5 WARNINGS AND PRECAUTIONS5.1 Suicidal Thoughts and Behaviors in Adolescents and Young Adults - In pooled analyses of placebo-controlled trials of antidepressant drugs (SSRIs and other antidepressant classes) that ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described in more detail in other sections of the prescribing information: Hypersensitivity [see Contraindications (4)] Suicidal Thoughts and Behaviors [see ...
-
7 DRUG INTERACTIONSTable 5 includes clinically important drug interactions with mirtazapine tablets [see Clinical Pharmacology (12.3)]. Table 5: Clinically Important Drug Interactions with Mirtazapine ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antidepressants during pregnancy. Healthcare providers ...
-
10 OVERDOSAGEHuman Experience - In premarketing clinical studies, there were reports of mirtazapine tablet overdose alone or in combination with other pharmacological agents. Signs and symptoms reported in ...
-
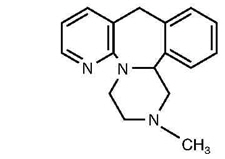
11 DESCRIPTIONMirtazapine tablets, USP contain mirtazapine, USP. Mirtazapine has a tetracyclic chemical structure and belongs to the piperazino-azepine group of compounds. It is designated ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of mirtazapine for the treatment of major depressive disorder, is unclear. However, its efficacy could be mediated through its activity as an ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Carcinogenicity studies were conducted with mirtazapine given in the diet at doses of 2, 20, and 200 mg/kg/day to mice ...
-
14 CLINICAL STUDIESThe efficacy of mirtazapine tablets as a treatment for major depressive disorder was established in 4 placebo-controlled, 6-week trials in adult outpatients meeting DSM-III criteria for major ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGMirtazapine tablets, USP are supplied as: Tablet Strength Tablet Color/Shape Tablet MarkingsPackage Configuration NDC Code - 7.5 mgOrange, oval tabletFilm coated tablet debossed with ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Suicidal Thoughts and Behaviors - Advise patients and caregivers to look for the emergence of suicidality ...
-
SPL UNCLASSIFIED SECTIONDispense with Medication Guide available at www.prasco.com/medguide/mirtazapine - Made in Malta - Manufactured for: Prasco, LLC - Mason, OH 45040 USA - Revised: 02/2023 - Part code: 2907-01
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration. Revised 02/2023 - MEDICATION GUIDE - MIRTAZAPINE TABLETS - (mir taz' a ...
-
PRINCIPAL DISPLAY PANEL - 7.5 mg Tablet Bottle Label 30 CTNDC 66993-606-30 - Hampton Laine - Mirtazapine Tablets, USP - 7.5 mg - PHARMACIST: Dispense with Medication Guide available at: www.prasco.com/medguide/mirtazapine - 30 Tablets - Rx only
-
PRINCIPAL DISPLAY PANEL - 7.5 mg Tablet Bottle Label 500 CTNDC 66993-606-04 - Hampton Laine - Mirtazapine Tablets, USP - 7.5 mg - PHARMACIST: Dispense with Medication Guide available at: www.prasco.com/medguide/mirtazapine - 500 Tablets - Rx only
-
PRINCIPAL DISPLAY PANEL - 15 mg Tablet Bottle Label 30 CTNDC 66993-607-30 - Hampton Laine - Mirtazapine Tablets, USP - 15 mg - PHARMACIST: Dispense with Medication Guide available at: www.prasco.com/medguide/mirtazapine - 30 Tablets - Rx only
-
PRINCIPAL DISPLAY PANEL - 15 mg Tablet Bottle Label 500 CTNDC 66993-607-04 - Hampton Laine - Mirtazapine Tablets, USP - 15 mg - PHARMACIST: Dispense with Medication Guide available at: www.prasco.com/medguide/mirtazapine - 500 Tablets - Rx only
-
PRINCIPAL DISPLAY PANEL - 30 mg Tablet Bottle Label 30 CTNDC 66993-608-30 - Hampton Laine - Mirtazapine Tablets, USP - 30 mg - PHARMACIST: Dispense with Medication Guide available at: www.prasco.com/medguide/mirtazapine - 30 Tablets - Rx only
-
PRINCIPAL DISPLAY PANEL - 30 mg Tablet Bottle Label 500 CTNDC 66993-608-04 - Hampton Laine - Mirtazapine Tablets, USP - 30 mg - PHARMACIST: Dispense with Medication Guide available at: www.prasco.com/medguide/mirtazapine - 500 Tablets - Rx only
-
PRINCIPAL DISPLAY PANEL - 45 mg Tablet Bottle Label 30 CTNDC 66993-609-30 - Hampton Laine - Mirtazapine Tablets, USP - 45 mg - PHARMACIST: Dispense with Medication Guide available at: www.prasco.com/medguide/mirtazapine - 30 Tablets - Rx only
-
PRINCIPAL DISPLAY PANEL - 45 mg Tablet Bottle Label 500 CTNDC 66993-609-04 - Hampton Laine - Mirtazapine Tablets, USP - 45 mg - PHARMACIST: Dispense with Medication Guide available at: www.prasco.com/medguide/mirtazapine - 500 Tablets - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information