Label: DAPIPRAZOLE kit
- NDC Code(s): 53020-245-01, 53020-255-01, 53020-265-01
- Packager: Baradaina, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION:
For ophthalmic use only.
Dapiprazole hydrochloride is an alpha-adrenergic blocking agent.
Dapiprazole hydrochloride is 5,6,7,8-tetrahydro-3-[2-(4- o .tolyl-1-piperazinyl)ethyl]- s -triazolo[4,3-a]pyridine hydrochloride.
Dapiprazole hydrochloride has the empirical formula C 19H 27N 5 • HCl and a molecular weight of 361.93.
The structural formula for dapiprazole hydrochloride is:
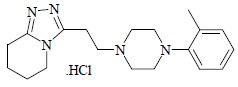
Dapiprazole hydrochloride is a sterile, white, lyophilized powder soluble in water.
Dapiprazole hydrochloride ophthalmic solution, 0.5% is a clear, colorless, slightly viscous solution for topical application. Each mL (when reconstituted as directed) contains 5 mg of dapiprazole hydrochloride as the active ingredient.
The reconstituted solution has a pH of approximately 6.6 and an osmolarity of approximately 415 mOsm.
The inactive ingredients include: mannitol (2%), sodium chloride, hydroxypropyl methylcellulose (0.4%), edetate sodium (0.01%), sodium phosphate dibasic, sodium phosphate monobasic, water for injection, and benzalkonium chloride (0.01%) as a preservative.
Dapiprazole hydrochloride ophthalmic solution, 0.5% is supplied in a kit consisting of one vial of dapiprazole hydrochloride (25 mg), one vial of diluent (5 mL) and one dropper for dispensing.
-
CLINICAL PHARMACOLOGY:
Dapiprazole hydrochloride ophthalmic solution acts through blocking the alpha-adrenergic receptors in smooth muscle. Dapiprazole hydrochloride ophthalmic solution produces miosis through an effect on the dilator muscle of the iris.
Dapiprazole hydrochloride ophthalmic solution does not have any significant activity on ciliary muscle contraction and, therefore does not induce a significant change in the anterior chamber depth or the thickness of the lens.
Dapiprazole hydrochloride ophthalmic solution has demonstrated safe and rapid reversal of mydriasis produced by phenylephrine and to a lesser degree tropicamide. In patients with decreased accommodative amplitude due to treatment with tropicamide, dapiprazole hydrochloride ophthalmic solution partially restores the accommodative amplitude. This activity is not only due to its miotic effect but also to a direct effect on accommodation.
Eye color affects the rate of pupillary constriction. In individuals with brown irides, the rate of pupillary constriction may be slightly slower than in individuals with blue or green irides. Eye color does not appear to affect the final pupil size.
Dapiprazole hydrochloride ophthalmic solution does not significantly alter intraocular pressure in normotensive eyes or in eyes with elevated intraocular pressure.
-
INDICATIONS AND USAGE:
Dapiprazole hydrochloride ophthalmic solution is indicated in the treatment of iatrogenically induced mydriasis produced by adrenergic (phenylephrine) or parasympatholytic (tropicamide) agents. Dapiprazole hydrochloride ophthalmic solution is not indicated for the reduction of intraocular pressure or in the treatment of open angle glaucoma.
- CONTRAINDICATIONS:
- WARNING:
-
PRECAUTIONS:
Information to Patients:
Miosis may cause difficulty in dark adaptation and may reduce the field of vision. Patients should exercise caution when involved in night driving or other activities in poor illumination.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Dapiprazole has been shown to significantly increase the incidence of liver tumors in rats after continuous dietary administration for 104 weeks. This effect was found only in male rats treated with the highest dose administered in the study, i.e., 300 mg/kg/day, (80,000 times the human dose) and was not observed in male and female rats at doses of 30 and 100 mg/kg/day and female rats at doses of 300 mg/kg/day.
Negative results have been reported on the mutagenicity and impairment of fertility studies with dapiprazole hydrochloride.
Pregnancy:
Reproduction studies have been performed in rats and rabbits at doses up to 128,000 (rat) and 27,000 (rabbit) times the human ophthalmic dose and revealed no evidence of impaired fertility or harm to the fetus due to dapiprazole hydrochloride. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
-
ADVERSE REACTIONS:
In controlled studies the most frequent reaction to dapiprazole was conjunctival injection lasting 20 minutes in over 80% of patients. Burning on instillation of dapiprazole hydrochloride ophthalmic solution was reported in approximately half of all patients. Reactions occurring in 10% to 40% of patients included ptosis, lid erythema, lid edema, chemosis, itching, punctate keratitis, corneal edema, browache, photophobia and headaches. Other reactions reported less frequently included dryness of eyes, tearing and blurring of vision.
-
DOSAGE AND ADMINISTRATION:
Two drops followed 5 minutes later by an additional 2 drops applied topically to the conjunctiva of each eye should be administered after the ophthalmic examination to reverse the diagnostic mydriasis. Dapiprazole hydrochloride ophthalmic solution should not be used in the same patient more frequently than once per week.
Directions for Preparing Eyedrops:
- Use aseptic technique.
- Tear off aluminum seals, remove and discard rubber plugs from both drug and diluent vials
- Pour diluent into drug vial.
- Remove dropper assembly from its sterile wrapping and attach to the drug vial.
- Shake container for several minutes to ensure mixing.
- HOW SUPPLIED:
-
Storage and Stability of Eyedrops:
Once the ophthalmic solution has been reconstituted it may be stored at 20° - 25°C (68° - 77°F) [See USP Controlled Temperature] for 21 days. Discard any solution that is not clear and colorless.
Updated: February 2019
Manufactured by:
Exela Pharma Sciences, LLC
Lenoir, NC 28645Manufactured For:
Baradaina, LLC
8780 W. Golf Road, Suite 304
Niles , Illinois 60714Rx only
- PRINCIPAL DISPLAY-Container Label
-
INGREDIENTS AND APPEARANCE
DAPIPRAZOLE
dapiprazole kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:53020-265 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53020-265-01 1 in 1 CARTON; Type 1: Convenience Kit of Co-Package 07/31/2019 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 5 mL Part 2 1 VIAL 5 mL Part 1 of 2 DAPIPRAZOLE
dapiprazole powder, for solutionProduct Information Item Code (Source) NDC:53020-255 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DAPIPRAZOLE HYDROCHLORIDE (UNII: DS9UJN1I0X) (DAPIPRAZOLE - UNII:5RNZ8GJO7K) DAPIPRAZOLE HYDROCHLORIDE 25 mg in 5 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53020-255-01 5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204902 07/31/2019 Part 2 of 2 DILUENT
diluent solutionProduct Information Item Code (Source) NDC:53020-245 Route of Administration OPHTHALMIC Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYPROMELLOSE 2208 (100 MPA.S) (UNII: B1QE5P712K) EDETATE SODIUM (UNII: MP1J8420LU) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) WATER (UNII: 059QF0KO0R) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53020-245-01 5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204902 07/31/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204902 07/31/2019 Labeler - Baradaina, LLC (078529962) Registrant - Baradaina, LLC (078529962)


