Label: PAXLOVID- nirmatrelvir and ritonavir kit
-
NDC Code(s):
0069-0521-11,
0069-1345-11,
0069-1735-05,
0069-1735-11, view more0069-2085-02, 0069-2085-06, 0069-2085-11, 0069-5045-06, 0069-5045-30, 0069-5317-02, 0069-5317-20, 0069-5321-03, 0069-5321-30
- Packager: Pfizer Laboratories Div Pfizer Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 15, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PAXLOVID safely and effectively. See full prescribing information for PAXLOVID. PAXLOVIDTM (nirmatrelvir tablets; ritonavir ...These highlights do not include all the information needed to use PAXLOVID safely and effectively. See full prescribing information for PAXLOVID.
PAXLOVIDTM (nirmatrelvir tablets; ritonavir tablets), co-packaged for oral use
Initial U.S. Approval: 2023WARNING: SIGNIFICANT DRUG INTERACTIONS WITH PAXLOVID
See full prescribing information for complete boxed warning.
- •
- PAXLOVID includes ritonavir, a strong CYP3A inhibitor, which may lead to greater exposure of certain concomitant medications, resulting in potentially severe, life-threatening, or fatal events. (4, 5.1, 7)
- •
- Prior to prescribing PAXLOVID: 1) Review all medications taken by the patient to assess potential drug-drug interactions with a strong CYP3A inhibitor like PAXLOVID and 2) Determine if concomitant medications require a dose adjustment, interruption, and/or additional monitoring. (7)
- •
- Consider the benefit of PAXLOVID treatment in reducing hospitalization and death, and whether the risk of potential drug-drug interactions for an individual patient can be appropriately managed. (5.1, 7, 14)
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
PAXLOVID which includes nirmatrelvir, a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) main protease (Mpro: also referred to as 3CLpro or nsp5 protease) inhibitor, and ritonavir, an HIV-1 protease inhibitor and CYP3A inhibitor, is indicated for the treatment of mild-to-moderate coronavirus disease 2019 (COVID-19) in adults who are at high risk for progression to severe COVID-19, including hospitalization or death. (1)
Limitations of UsePAXLOVID is not approved for use as pre-exposure or post-exposure prophylaxis for prevention of COVID-19. (1)
DOSAGE AND ADMINISTRATION
PAXLOVID is nirmatrelvir tablets co-packaged with ritonavir tablets. (2.1)
Nirmatrelvir must be co-administered with ritonavir. (2.1)
- •
- Initiate PAXLOVID treatment as soon as possible after diagnosis of COVID-19 and within 5 days of symptom onset. (2.1)
- •
- Administer orally with or without food. (2.1)
- •
- Administer at approximately the same time each day. (2.2, 2.3)
- •
- Dosage: 300 mg nirmatrelvir (two 150 mg tablets) with 100 mg ritonavir (one 100 mg tablet), with all 3 tablets taken together twice daily for 5 days. (2.2)
- •
- Dose Reduction for Renal Impairment (2.3, 8.6, 12.3)
Abbreviation: eGFR=estimated glomerular filtration rate. - Renal Function
Days of Treatment
- Dose and Dose Frequency*
- Moderate renal impairment (eGFR ≥30 to <60 mL/min)
- Days 1-5
- 150 mg nirmatrelvir (one 150 mg tablet) with 100 mg ritonavir (one 100 mg tablet) twice daily
- Severe renal impairment (eGFR <30 mL/min) including those requiring hemodialysis†
- Day 1
- 300 mg nirmatrelvir (two 150 mg tablets) with 100 mg ritonavir (one 100 mg tablet) once
- Days 2-5
- 150 mg nirmatrelvir (one 150 mg tablet) with 100 mg ritonavir (one 100 mg tablet) once daily
CONTRAINDICATIONS
- •
- History of clinically significant hypersensitivity reactions to the active ingredients (nirmatrelvir or ritonavir) or any other components. (4)
- •
- Co-administration with drugs highly dependent on CYP3A for clearance and for which elevated concentrations are associated with serious and/or life-threatening reactions. (4, 7.3)
- •
- Co-administration with potent CYP3A inducers where significantly reduced nirmatrelvir or ritonavir plasma concentrations may be associated with the potential for loss of virologic response and possible resistance. (4)
WARNINGS AND PRECAUTIONS
- •
- The concomitant use of PAXLOVID and certain other drugs may result in potentially significant drug interactions. Consult the Full Prescribing Information prior to and during treatment for potential drug interactions. (5.1, 7)
- •
- Hypersensitivity Reactions: Anaphylaxis, serious skin reactions (including toxic epidermal necrolysis and Stevens-Johnson syndrome), and other hypersensitivity reactions have been reported with PAXLOVID. If signs and symptoms of a clinically significant hypersensitivity reaction or anaphylaxis occur, immediately discontinue PAXLOVID and initiate appropriate medications and/or supportive care. (5.2)
- •
- Hepatotoxicity: Hepatic transaminase elevations, clinical hepatitis, and jaundice have occurred in patients receiving ritonavir. (5.3)
- •
- HIV-1 Drug Resistance: PAXLOVID use may lead to a risk of HIV-1 developing resistance to HIV protease inhibitors in individuals with uncontrolled or undiagnosed HIV-1 infection. (5.4)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥1% and greater incidence than in the placebo group) are dysgeusia and diarrhea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Co-administration of PAXLOVID can alter the plasma concentrations of other drugs and other drugs may alter the plasma concentrations of PAXLOVID. Consider the potential for drug interactions prior to and during PAXLOVID therapy and review concomitant medications during PAXLOVID therapy. (4, 5.1, 7, 12.3)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2025
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: SIGNIFICANT DRUG INTERACTIONS WITH PAXLOVID
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Information
2.2 Recommended Dosage
2.3 Dosage in Patients with Renal Impairment
2.4 Use in Patients with Hepatic Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Serious Adverse Reactions Due to Drug Interactions
5.2 Hypersensitivity Reactions
5.3 Hepatotoxicity
5.4 Risk of HIV-1 Resistance Development
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Potential for PAXLOVID to Affect Other Drugs
7.2 Potential for Other Drugs to Affect PAXLOVID
7.3 Established and Other Potentially Significant Drug Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Efficacy in Subjects at High Risk of Progression to Severe COVID-19 (EPIC-HR)
14.2 Trial in Unvaccinated Subjects Without a Risk Factor for Progression to Severe COVID-19 or Subjects Fully Vaccinated Against COVID-19 With at Least One Factor for Progression to Severe COVID-19 (EPIC-SR)
14.3 Post-Exposure Prophylaxis Trial
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: SIGNIFICANT DRUG INTERACTIONS WITH PAXLOVID
- •
- PAXLOVID includes ritonavir, a strong CYP3A inhibitor, which may lead to greater exposure of certain concomitant medications, resulting in potentially severe, life-threatening, or fatal events [see Contraindications (4), Warnings and Precautions (5.1), and Drug Interactions (7)].
- •
- Prior to prescribing PAXLOVID: 1) Review all medications taken by the patient to assess potential drug-drug interactions with a strong CYP3A inhibitor like PAXLOVID and 2) Determine if concomitant medications require a dose adjustment, interruption, and/or additional monitoring [see Drug Interactions (7)].
- •
- Consider the benefit of PAXLOVID treatment in reducing hospitalization and death, and whether the risk of potential drug-drug interactions for an individual patient can be appropriately managed [see Warnings and Precautions (5.1), Drug Interactions (7), and Clinical Studies (14)].
-
1 INDICATIONS AND USAGEPAXLOVID is indicated for the treatment of mild-to-moderate coronavirus disease 2019 (COVID-19) in adults who are at high risk for progression to severe COVID-19, including hospitalization or ...
PAXLOVID is indicated for the treatment of mild-to-moderate coronavirus disease 2019 (COVID-19) in adults who are at high risk for progression to severe COVID-19, including hospitalization or death.
CloseLimitations of Use
PAXLOVID is not approved for use as pre-exposure or post-exposure prophylaxis for prevention of COVID-19 [see Clinical Studies (14.3)].
-
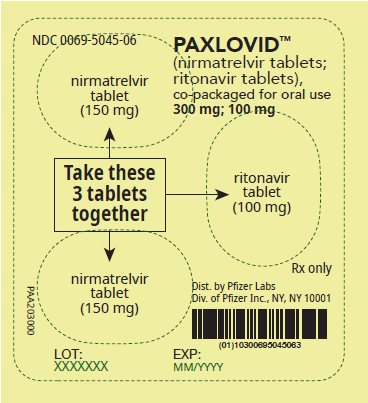
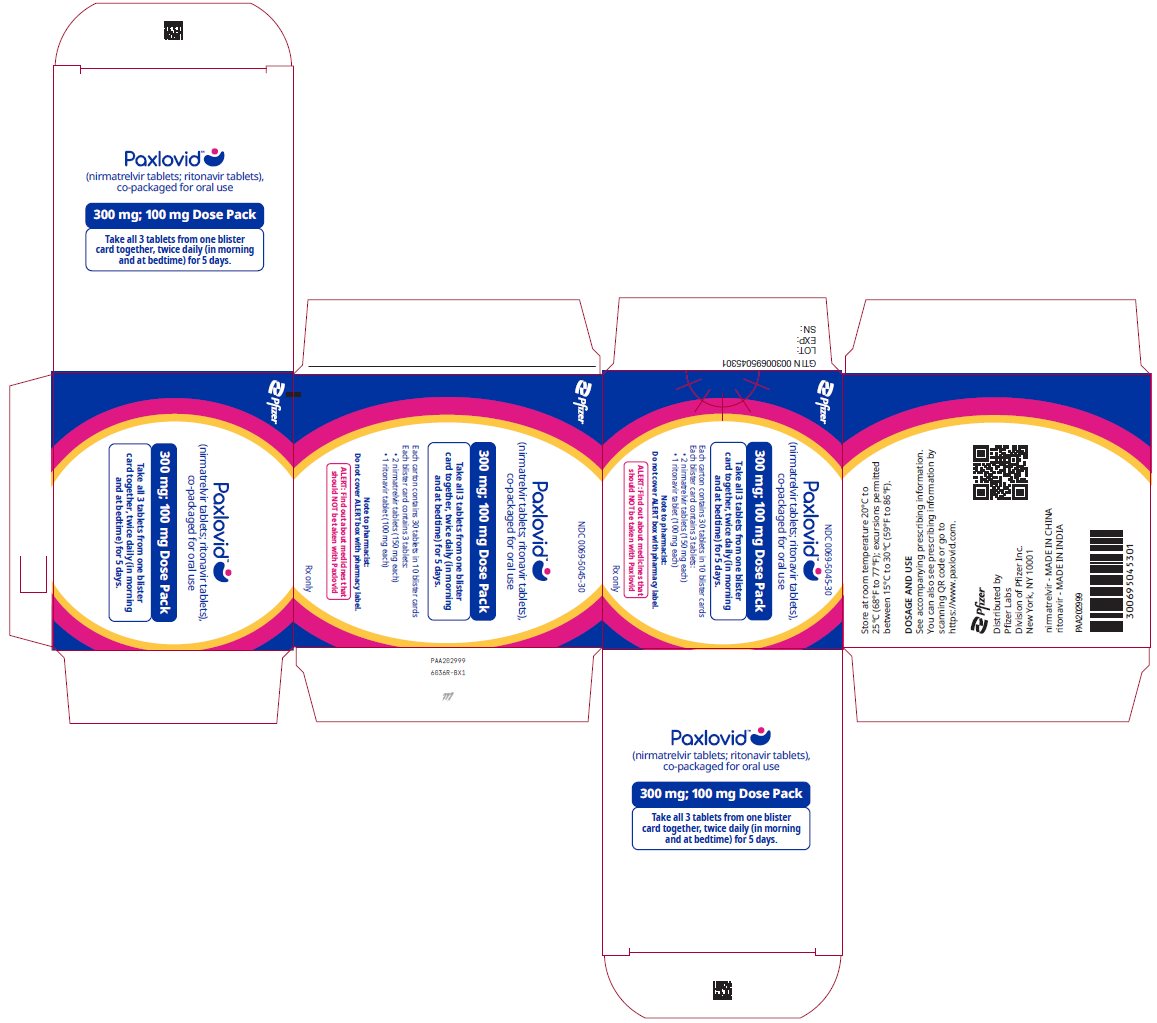
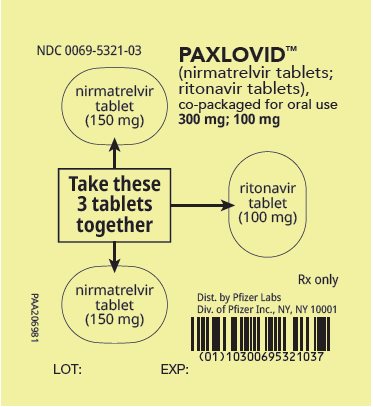
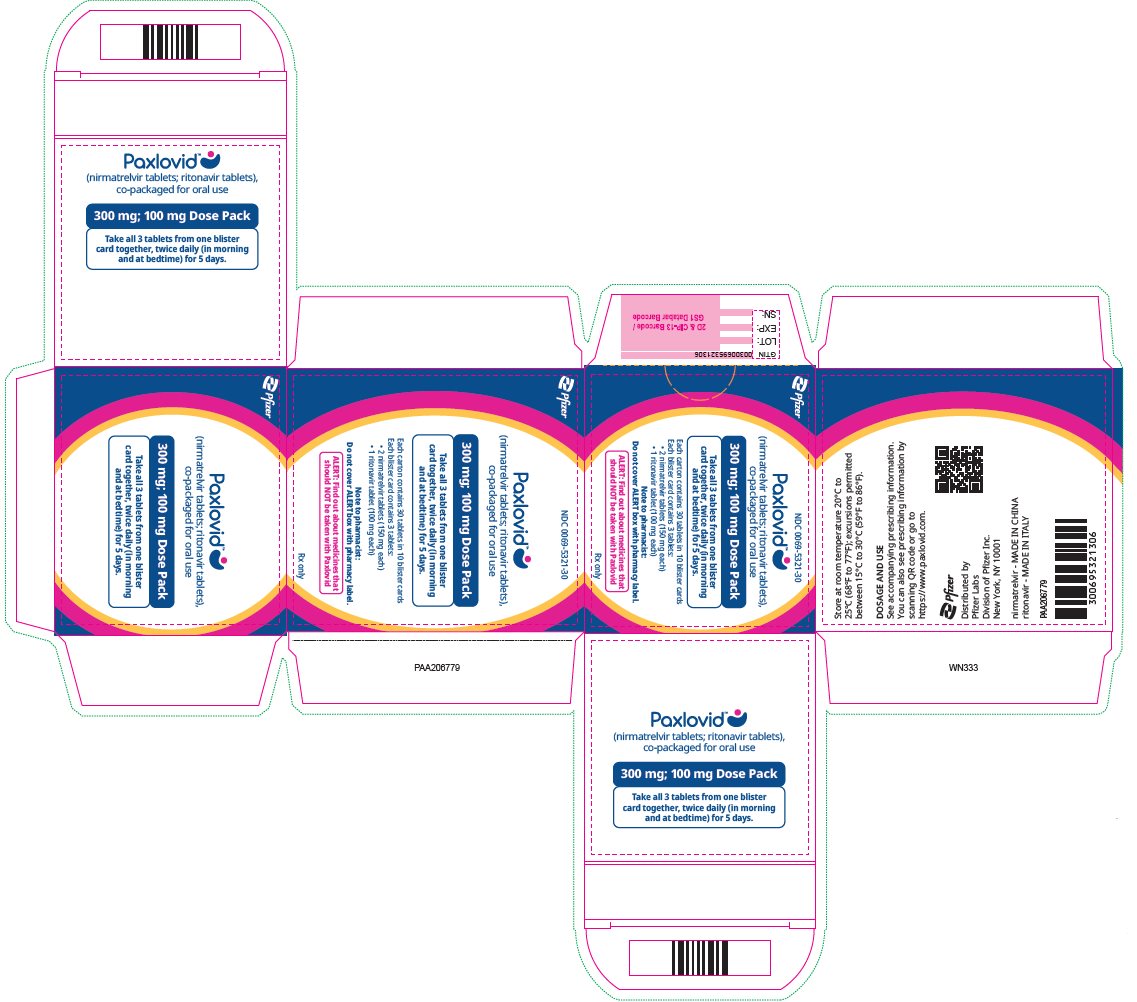
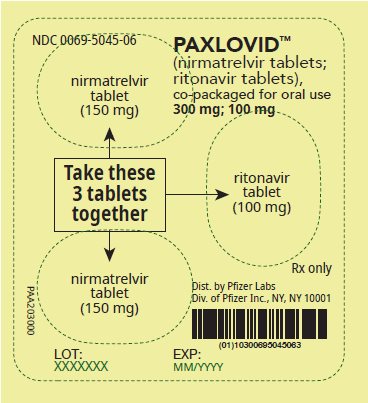
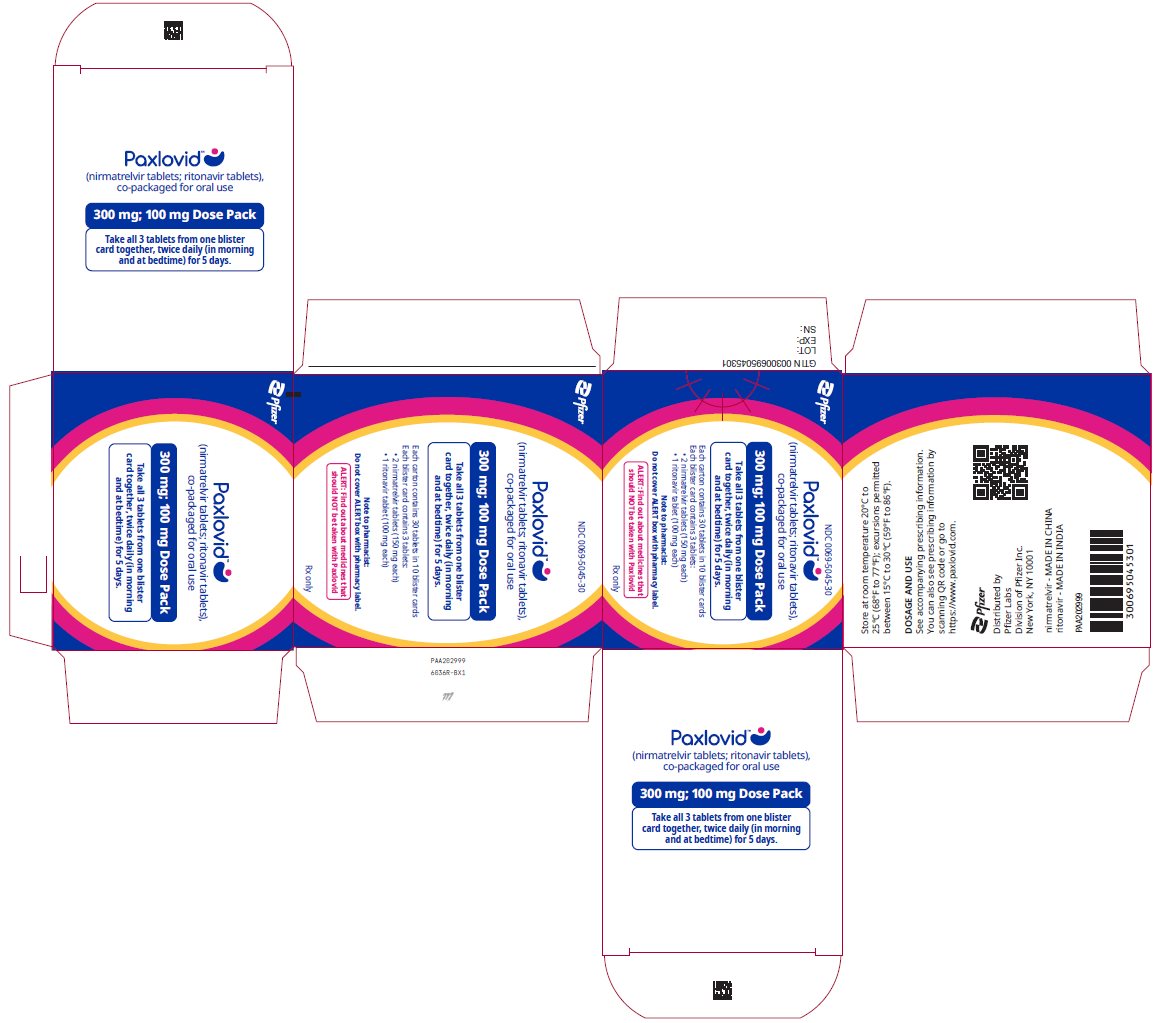
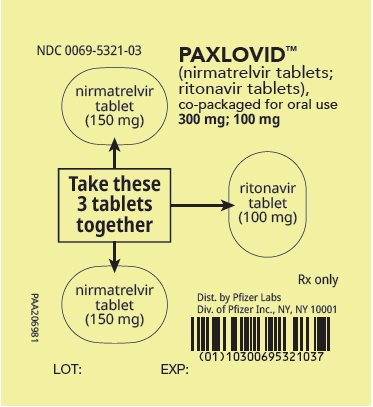
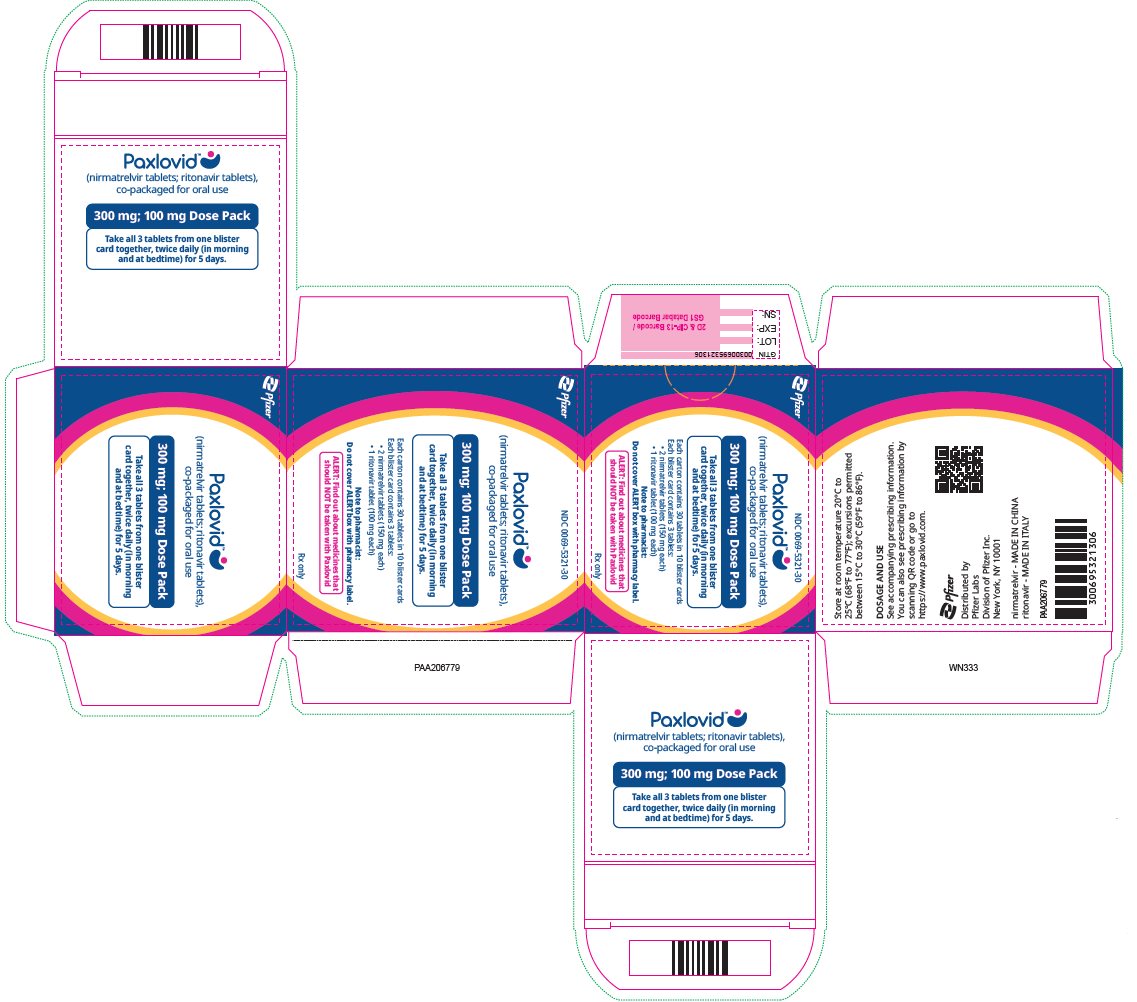
2 DOSAGE AND ADMINISTRATION2.1 Important Dosage and Administration Information - PAXLOVID is nirmatrelvir tablets co-packaged with ritonavir tablets. There are three different dose packs available: • PAXLOVID ...
2.1 Important Dosage and Administration Information
PAXLOVID is nirmatrelvir tablets co-packaged with ritonavir tablets. There are three different dose packs available:
- •
- PAXLOVID (nirmatrelvir; ritonavir) co-packaged for oral use 300 mg;100 mg [see Dosage and Administration (2.2)].
- •
- PAXLOVID (nirmatrelvir; ritonavir) co-packaged for oral use 150 mg;100 mg for patients with moderate renal impairment [see Dosage and Administration (2.3)].
- •
- PAXLOVID (nirmatrelvir; ritonavir) co-packaged for oral use 300 mg;100 mg (Day 1) and 150 mg;100 mg (Days 2-5) for patients with severe renal impairment [see Dosage and Administration (2.3)].
Nirmatrelvir must be co-administered with ritonavir. Failure to correctly co-administer nirmatrelvir with ritonavir may result in plasma levels of nirmatrelvir that are insufficient to achieve the desired therapeutic effect.
Prescriptions should specify the numeric dose of each active ingredient within PAXLOVID [see Dosage and Administration (2.2, 2.3)]. Completion of the full 5-day treatment course and continued isolation in accordance with public health recommendations are important to maximize viral clearance and minimize transmission of SARS-CoV-2.
The 5-day treatment course of PAXLOVID should be initiated as soon as possible after a diagnosis of COVID-19 has been made, and within 5 days of symptom onset even if baseline COVID-19 symptoms are mild. Should a patient require hospitalization due to severe or critical COVID-19 after starting treatment with PAXLOVID, the patient should complete the full 5-day treatment course per the healthcare provider's discretion.
If the patient misses a dose of PAXLOVID within 8 hours of the time it is usually taken, the patient should take it as soon as possible and resume the normal dosing schedule. If the patient misses a dose by more than 8 hours, the patient should not take the missed dose and instead take the next dose at the regularly scheduled time. The patient should not double the dose to make up for a missed dose.
PAXLOVID (both nirmatrelvir and ritonavir tablets) can be taken with or without food [see Clinical Pharmacology (12.3)]. The tablets should be swallowed whole and not chewed, broken, or crushed.
2.2 Recommended Dosage
The recommended dosage for PAXLOVID is 300 mg nirmatrelvir (two 150 mg tablets) with 100 mg ritonavir (one 100 mg tablet) with all 3 tablets taken together orally twice daily in the morning and at bedtime for 5 days.
2.3 Dosage in Patients with Renal Impairment
Prescriptions should specify the numeric dose of each active ingredient within PAXLOVID. Providers should counsel patients about renal dosing instructions [see Patient Counseling Information (17)].
No dosage adjustment is recommended in patients with mild renal impairment [estimated glomerular filtration rate (eGFR) ≥60 to <90 mL/min].
In patients with moderate renal impairment (eGFR ≥30 to <60 mL/min) or with severe renal impairment (eGFR <30 mL/min) including those requiring hemodialysis, the dosage of PAXLOVID should be reduced as shown in Table 1. PAXLOVID should be administered at approximately the same time each day for 5 days. On days patients with severe renal impairment undergo hemodialysis, the PAXLOVID dose should be administered after hemodialysis [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3), and How Supplied/Storage and Handling (16)].
Table 1: Recommended Dose and Regimen for Patients with Renal Impairment
Abbreviation: eGFR=estimated glomerular filtration rate. Renal Function
Days of Treatment
Dose and Dose Frequency*
Moderate renal impairment (eGFR ≥30 to <60 mL/min)
Days 1-5
150 mg nirmatrelvir (one 150 mg tablet) with 100 mg ritonavir (one 100 mg tablet) twice daily
Severe renal impairment (eGFR <30 mL/min) including those requiring hemodialysis†
Day 1
300 mg nirmatrelvir (two 150 mg tablets) with 100 mg ritonavir (one 100 mg tablet) once
Days 2-5
150 mg nirmatrelvir (one 150 mg tablet) with 100 mg ritonavir (one 100 mg tablet) once daily
Close2.4 Use in Patients with Hepatic Impairment
No dosage adjustment is needed in patients with mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment.
No pharmacokinetic or safety data are available regarding the use of nirmatrelvir or ritonavir in subjects with severe (Child-Pugh Class C) hepatic impairment; therefore, PAXLOVID is not recommended for use in patients with severe hepatic impairment [see Use in Specific Populations (8.7)].
-
3 DOSAGE FORMS AND STRENGTHSPAXLOVID is nirmatrelvir tablets co-packaged with ritonavir tablets [see How Supplied/Storage and Handling (16)]. • Nirmatrelvir is supplied as oval, pink immediate-release, film-coated tablets ...
PAXLOVID is nirmatrelvir tablets co-packaged with ritonavir tablets [see How Supplied/Storage and Handling (16)].
- •
- Nirmatrelvir is supplied as oval, pink immediate-release, film-coated tablets debossed with "PFE" on one side and "3CL" on the other side. Each tablet contains 150 mg of nirmatrelvir.
- •
- Ritonavir is supplied as white or white to off-white film-coated tablets uniquely identified by the color, shape, and debossing. Each tablet contains 100 mg of ritonavir.
-
4 CONTRAINDICATIONSPAXLOVID is contraindicated in patients with a history of clinically significant hypersensitivity reactions [e.g., toxic epidermal necrolysis (TEN) or Stevens-Johnson syndrome] to its active ...
PAXLOVID is contraindicated in patients with a history of clinically significant hypersensitivity reactions [e.g., toxic epidermal necrolysis (TEN) or Stevens-Johnson syndrome] to its active ingredients (nirmatrelvir or ritonavir) or any other components of the product.
PAXLOVID is contraindicated with drugs that are primarily metabolized by CYP3A and for which elevated concentrations are associated with serious and/or life-threatening reactions and drugs that are strong CYP3A inducers where significantly reduced nirmatrelvir or ritonavir plasma concentrations may be associated with the potential for loss of virologic response and possible resistance. There are certain other drugs for which concomitant use with PAXLOVID should be avoided and/or dose adjustment, interruption, or therapeutic monitoring is recommended. Drugs listed in this section are a guide and not considered a comprehensive list of all drugs that may be contraindicated with PAXLOVID. The healthcare provider should consult other appropriate resources such as the prescribing information for the interacting drug for comprehensive information on dosing or monitoring with concomitant use of a strong CYP3A inhibitor like PAXLOVID [see Drug Interactions (7.3)]:
- ➢
- Drugs that are primarily metabolized by CYP3A for which elevated concentrations are associated with serious and/or life-threatening reactions [see Drug Interactions (7.3)]:
- •
- Alpha 1-adrenoreceptor antagonist: alfuzosin
- •
- Antianginal: ranolazine
- •
- Antiarrhythmic: amiodarone, dronedarone, flecainide, propafenone, quinidine
- •
- Anti-gout: colchicine (in patients with renal and/or hepatic impairment [see Table 2, Drug Interactions (7.3)])
- •
- Antipsychotics: lurasidone, pimozide
- •
- Benign prostatic hyperplasia agents: silodosin
- •
- Cardiovascular agents: eplerenone, ivabradine
- •
- Ergot derivatives: dihydroergotamine, ergotamine, methylergonovine
- •
- HMG-CoA reductase inhibitors: lovastatin, simvastatin (these drugs can be temporarily discontinued to allow PAXLOVID use [see Table 2, Drug Interactions (7.3)])
- •
- Immunosuppressants: voclosporin
- •
- Microsomal triglyceride transfer protein inhibitor: lomitapide
- •
- Migraine medications: eletriptan, ubrogepant
- •
- Mineralocorticoid receptor antagonists: finerenone
- •
- Opioid antagonists: naloxegol
- •
- PDE5 inhibitor: sildenafil (Revatio®) when used for pulmonary arterial hypertension (PAH)
- •
- Sedative/hypnotics: triazolam, oral midazolam
- •
- Serotonin receptor 1A agonist/serotonin receptor 2A antagonist: flibanserin
- •
- Vasopressin receptor antagonists: tolvaptan
- ➢
- Drugs that are strong CYP3A inducers where significantly reduced nirmatrelvir or ritonavir plasma concentrations may be associated with the potential for loss of virologic response and possible resistance. PAXLOVID cannot be started immediately after discontinuation of any of the following medications due to the delayed offset of the recently discontinued CYP3A inducer [see Drug Interactions (7.3)]:
- •
- Anticancer drugs: apalutamide, enzalutamide
- •
- Anticonvulsant: carbamazepine, phenobarbital, primidone, phenytoin
- •
- Antimycobacterials: rifampin, rifapentine
- •
- Cystic fibrosis transmembrane conductance regulator potentiators: lumacaftor/ivacaftor
- •
- Herbal products: St. John's Wort (hypericum perforatum)
-
5 WARNINGS AND PRECAUTIONS5.1 Risk of Serious Adverse Reactions Due to Drug Interactions - Initiation of PAXLOVID, which contains ritonavir, a strong CYP3A inhibitor, in patients receiving medications metabolized by CYP3A ...
5.1 Risk of Serious Adverse Reactions Due to Drug Interactions
Initiation of PAXLOVID, which contains ritonavir, a strong CYP3A inhibitor, in patients receiving medications metabolized by CYP3A or initiation of medications metabolized by CYP3A in patients already receiving PAXLOVID, may increase plasma concentrations of medications metabolized by CYP3A. Medications that induce CYP3A may decrease concentrations of PAXLOVID. These interactions may lead to:
- •
- Clinically significant adverse reactions, potentially leading to severe, life-threatening, or fatal events from greater exposures of concomitant medications.
- •
- Loss of therapeutic effect of PAXLOVID and possible development of viral resistance.
Severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with PAXLOVID. The most commonly reported concomitant medications resulting in serious adverse reactions were calcineurin inhibitors (e.g., tacrolimus, cyclosporine), followed by calcium channel blockers.
Prior to prescribing PAXLOVID, review all medications taken by the patient to assess potential drug-drug interactions and determine if concomitant medications require a dose adjustment, interruption, and/or additional monitoring (e.g., calcineurin inhibitors) [see Contraindications (4) and Drug Interactions (7)]. See Table 2 for clinically significant drug interactions, including contraindicated drugs. Drugs listed in Table 2 are a guide and not considered a comprehensive list of all possible drugs that may interact with PAXLOVID.
Consider the benefit of PAXLOVID treatment in reducing hospitalization and death, and whether the risk of potential drug-drug interactions for an individual patient can be appropriately managed [see Drug Interactions (7) and Clinical Studies (14)].
5.2 Hypersensitivity Reactions
Anaphylaxis, serious skin reactions (including toxic epidermal necrolysis and Stevens-Johnson syndrome), and other hypersensitivity reactions have been reported with PAXLOVID [see Adverse Reactions (6.1)]. If signs and symptoms of a clinically significant hypersensitivity reaction or anaphylaxis occur, immediately discontinue PAXLOVID and initiate appropriate medications and/or supportive care.
5.3 Hepatotoxicity
Hepatic transaminase elevations, clinical hepatitis, and jaundice have occurred in patients receiving ritonavir. Therefore, caution should be exercised when administering PAXLOVID to patients with pre-existing liver diseases, liver enzyme abnormalities, or hepatitis.
Close5.4 Risk of HIV-1 Resistance Development
Because nirmatrelvir is co-administered with ritonavir, there may be a risk of HIV-1 developing resistance to HIV protease inhibitors in individuals with uncontrolled or undiagnosed HIV-1 infection [see Contraindications (4) and Drug Interactions (7)].
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: • Hypersensitivity reactions [see Warnings and Precautions (5.2)] 6.1 Clinical Trials ...
The following clinically significant adverse reactions are described elsewhere in the labeling:
- •
- Hypersensitivity reactions [see Warnings and Precautions (5.2)]
Close6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of PAXLOVID is based on two Phase 2/3 randomized, placebo-controlled trials in symptomatic adult subjects 18 years of age and older with a laboratory confirmed diagnosis of SARS-CoV-2 infection. Subjects in both studies received PAXLOVID (nirmatrelvir/ritonavir 300 mg/100 mg) or placebo every 12 hours for 5 days for the treatment of mild-to-moderate COVID-19 within 5 days of symptom onset [see Clinical Studies (14)]:
- •
- Trial C4671005 (EPIC-HR) enrolled subjects who were at high risk for progression to severe disease.
- •
- Trial C4671002 (EPIC-SR) enrolled subjects who were at standard risk for progression to severe disease (previously unvaccinated subjects at standard risk or fully vaccinated subjects with at least 1 risk factor for progression to severe disease).
Adverse reactions were those reported while subjects were on study medication and through 28 days after the last dose of study treatment.
In Trial C4671005 (EPIC-HR), 1,038 subjects received PAXLOVID and 1,053 subjects received placebo. The most common adverse reactions (≥1% incidence in the PAXLOVID group and occurring at a greater frequency than in the placebo group) were dysgeusia (5% and <1%, respectively) and diarrhea (3% and 2%, respectively).
Among vaccinated or unvaccinated subjects at standard risk or fully vaccinated subjects with at least 1 risk factor for progression to severe disease in Trial C4671002 (EPIC-SR), 540 subjects received PAXLOVID and 528 subjects received placebo. The adverse reactions observed were consistent with those observed in EPIC-HR.
Trial C4671028 (EPIC-SRI) was a Phase 1, open-label trial that evaluated the effects of severe renal impairment on the pharmacokinetics, safety, and tolerability of PAXLOVID in non-hospitalized adult participants with COVID-19 and severe renal impairment. A total of 15 subjects with severe renal impairment were enrolled in this trial, with 12 subjects receiving intermittent hemodialysis and 3 subjects not on hemodialysis. Subjects received nirmatrelvir/ritonavir 300 mg/100 mg once on Day 1 followed by nirmatrelvir/ritonavir 150 mg/100 mg once daily from Days 2-5. The safety profile of PAXLOVID in subjects with severe renal impairment, including those requiring hemodialysis, was consistent with the safety profile observed in the Phase 2/3 randomized, placebo-controlled trials.
Emergency Use Authorization Experience in Subjects with COVID-19
The following adverse reactions have been identified during use of PAXLOVID under Emergency Use Authorization.
Immune System Disorders: Anaphylaxis, hypersensitivity reactions [see Warnings and Precautions (5.2)]
Skin and Subcutaneous Tissue Disorders: Toxic epidermal necrolysis, Stevens-Johnson syndrome [see Warnings and Precautions (5.2)]
Nervous System Disorders: Headache
Vascular Disorders: Hypertension
Gastrointestinal Disorders: Abdominal pain, nausea, vomiting
General Disorders and Administration Site Conditions: Malaise
-
7 DRUG INTERACTIONS7.1 Potential for PAXLOVID to Affect Other Drugs - PAXLOVID (nirmatrelvir co-packaged with ritonavir) is a strong inhibitor of CYP3A, and an inhibitor of CYP2D6, P-gp and OATP1B1 ...
7.1 Potential for PAXLOVID to Affect Other Drugs
PAXLOVID (nirmatrelvir co-packaged with ritonavir) is a strong inhibitor of CYP3A, and an inhibitor of CYP2D6, P-gp and OATP1B1. Co-administration of PAXLOVID with drugs that are primarily metabolized by CYP3A and CYP2D6 or are transported by P-gp or OATP1B1 may result in increased plasma concentrations of such drugs and increase the risk of adverse events. Co-administration of PAXLOVID with drugs highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events is contraindicated [see Contraindications (4) and Drug Interactions (7.3) Table 2]. Co-administration with other CYP3A substrates may require a dose adjustment or additional monitoring as shown in Table 2.
7.2 Potential for Other Drugs to Affect PAXLOVID
Nirmatrelvir and ritonavir are CYP3A substrates; therefore, drugs that induce CYP3A may decrease nirmatrelvir and ritonavir plasma concentrations and reduce PAXLOVID therapeutic effect [see Contraindications (4) and Drug Interactions (7.3) Table 2].
Close7.3 Established and Other Potentially Significant Drug Interactions
Table 2 provides a listing of clinically significant drug interactions, including contraindicated drugs [see Contraindications (4) and Warnings and Precautions (5.1)]. Drugs listed in Table 2 are a guide and not considered a comprehensive list of all possible drugs that may interact with PAXLOVID. The healthcare provider should consult other appropriate resources such as the prescribing information for the interacting drug for comprehensive information on dosing or monitoring with concomitant use of a strong CYP3A inhibitor such as ritonavir.
Table 2: Established and Other Potentially Significant Drug Interactions Drug Class Drugs within Class Effect on Concentration Clinical Comments Alpha 1-
adrenoreceptor antagonistalfuzosin
↑ alfuzosin
Co-administration contraindicated due to potential hypotension [see Contraindications (4)].
Alpha 1-
adrenoreceptor antagonisttamsulosin
↑ tamsulosin
Avoid concomitant use with PAXLOVID.
Antianginal
ranolazine
↑ ranolazine
Co-administration contraindicated due to potential for serious and/or life-threatening reactions [see Contraindications (4)].
Antiarrhythmics
amiodarone,
dronedarone,
flecainide,
propafenone,
quinidine↑ antiarrhythmic
Co-administration contraindicated due to potential for cardiac arrhythmias [see Contraindications (4)].
Antiarrhythmics
lidocaine (systemic),
disopyramide↑ antiarrhythmic
Caution is warranted and therapeutic concentration monitoring is recommended for antiarrhythmics if available.
Anticancer drugs
apalutamide,
enzalutamide
↓ nirmatrelvir/ritonavir
Co-administration contraindicated due to potential loss of virologic response and possible resistance [see Contraindications (4)].
Anticancer drugs
abemaciclib,
ceritinib,
dasatinib,
encorafenib,
ibrutinib,
ivosidenib,
neratinib,
nilotinib,
venetoclax,
vinblastine,
vincristine↑ anticancer drugs
Avoid co-administration of encorafenib or ivosidenib due to potential risk of serious adverse events such as QT interval prolongation. Avoid use of neratinib, venetoclax or ibrutinib.
Co-administration of vincristine and vinblastine may lead to significant hematologic or gastrointestinal side effects.
For further information, refer to individual product label for anticancer drug.Anticoagulants
warfarin
↑↓ warfarin
Closely monitor international normalized ratio (INR) if co-administration with warfarin is necessary.
rivaroxaban
↑ rivaroxaban
Increased bleeding risk with rivaroxaban. Avoid concomitant use.
dabigatran*
↑ dabigatran
Increased bleeding risk with dabigatran. Depending on dabigatran indication and renal function, reduce dose of dabigatran or avoid concomitant use. Refer to the dabigatran product label for further information.
apixaban
↑ apixaban
Combined P-gp and strong CYP3A inhibitors increase blood levels of apixaban and increase the risk of bleeding. Dosing recommendations for co-administration of apixaban with PAXLOVID depend on the apixaban dose. Refer to the apixaban product label for more information.
Anticonvulsants
carbamazepine*,
phenobarbital,
primidone,
phenytoin↓ nirmatrelvir/ritonavir
Co-administration contraindicated due to potential loss of virologic response and possible resistance [see Contraindications (4)].
Anticonvulsants
clonazepam
↑ anticonvulsant
A dose decrease may be needed for clonazepam when co-administered with PAXLOVID and clinical monitoring is recommended.
Antidepressants
bupropion
↓ bupropion and active metabolite hydroxy-bupropion
Monitor for an adequate clinical response to bupropion.
trazodone
↑ trazodone
Adverse reactions of nausea, dizziness, hypotension, and syncope have been observed following co-administration of trazodone and ritonavir. A lower dose of trazodone should be considered. Refer to trazadone product label for further information.
Antifungals
voriconazole
↓ voriconazole
Avoid concomitant use of voriconazole.
ketoconazole,
isavuconazonium sulfate,
itraconazole*↑ ketoconazole
↑ isavuconazonium sulfate
↑ itraconazoleRefer to ketoconazole, isavuconazonium sulfate, and itraconazole product labels for further information.
↑ nirmatrelvir/ritonavir
A nirmatrelvir/ritonavir dose reduction is not needed.
Anti-gout
colchicine
↑ colchicine
Co-administration contraindicated due to potential for serious and/or life-threatening reactions in patients with renal and/or hepatic impairment [see Contraindications (4)].
Anti-HIV protease inhibitors
atazanavir,
darunavir,
tipranavir↑ protease inhibitor
For further information, refer to the respective protease inhibitors' prescribing information.
Patients on ritonavir- or cobicistat-containing HIV regimens should continue their treatment as indicated. Monitor for increased PAXLOVID or protease inhibitor adverse events.Anti-HIV
efavirenz,
maraviroc,
nevirapine,
zidovudine,
bictegravir/
emtricitabine/
tenofovir↑ efavirenz
↑ maraviroc
↑ nevirapine
↓ zidovudine
↑ bictegravir
↔ emtricitabine
↑ tenofovirFor further information, refer to the respective anti-HIV drugs prescribing information.
Anti-infective
clarithromycin,
erythromycin↑ clarithromycin
↑ erythromycinRefer to the respective prescribing information for anti-infective dose adjustment.
Antimycobacterial
rifampin,
rifapentine↓ nirmatrelvir/ritonavir
Co-administration contraindicated due to potential loss of virologic response and possible resistance. Alternate antimycobacterial drugs such as rifabutin should be considered [see Contraindications (4)].
Antimycobacterial
bedaquiline
↑ bedaquiline
Refer to the bedaquiline product label for further information.
rifabutin
↑ rifabutin
Refer to rifabutin product label for further information on rifabutin dose reduction.
Antipsychotics
lurasidone,
pimozide↑ lurasidone
↑ pimozideCo-administration contraindicated due to serious and/or life-threatening reactions such as cardiac arrhythmias [see Contraindications (4)].
Antipsychotics
quetiapine
↑ quetiapine
If co-administration is necessary, reduce quetiapine dose and monitor for quetiapine-associated adverse reactions. Refer to the quetiapine prescribing information for recommendations.
clozapine
↑ clozapine
If co-administration is necessary, consider reducing the clozapine dose and monitor for adverse reactions.
Benign prostatic hyperplasia agents
silodosin
↑ silodosin
Co-administration contraindicated due to potential for postural hypotension [see Contraindications (4)].
Calcium channel blockers
amlodipine,
diltiazem,
felodipine,
nicardipine,
nifedipine,
verapamil↑ calcium channel blocker
Caution is warranted and clinical monitoring of patients is recommended. A dose decrease may be needed for these drugs when co-administered with PAXLOVID.
If co-administered, refer to individual product label for calcium channel blocker for further information.Cardiac glycosides
digoxin
↑ digoxin
Caution should be exercised when co-administering PAXLOVID with digoxin, with appropriate monitoring of serum digoxin levels.
Refer to the digoxin product label for further information.Cardiovascular agents
eplerenone
↑ eplerenone
Co-administration with eplerenone is contraindicated due to potential for hyperkalemia [see Contraindications (4)].
ivabradine
↑ ivabradine
Co-administration with ivabradine is contraindicated due to potential for bradycardia or conduction disturbances [see Contraindications (4)].
Cardiovascular agents
aliskiren,
ticagrelor,
vorapaxar
clopidogrel↑ aliskiren
↑ ticagrelor
↑ vorapaxar
↓ clopidogrel active metaboliteAvoid concomitant use with PAXLOVID.
cilostazol
↑ cilostazol
Dosage adjustment of cilostazol is recommended. Refer to the cilostazol product label for more information.
Corticosteroids primarily metabolized by CYP3A
betamethasone,
budesonide,
ciclesonide,
dexamethasone,
fluticasone,
methylprednisolone,
mometasone,
triamcinolone↑ corticosteroid
Co-administration with corticosteroids (all routes of administration) of which exposures are significantly increased by strong CYP3A inhibitors can increase the risk for Cushing’s syndrome and adrenal suppression. However, the risk of Cushing’s syndrome and adrenal suppression associated with short-term use of a strong CYP3A inhibitor is low.
Alternative corticosteroids including beclomethasone, prednisone, and prednisolone should be considered.Cystic fibrosis transmembrane conductance regulator potentiators
lumacaftor/ivacaftor
↓ nirmatrelvir/ritonavir
Co-administration contraindicated due to potential loss of virologic response and possible resistance [see Contraindications (4)].
Cystic fibrosis transmembrane conductance regulator potentiators
ivacaftor
elexacaftor/tezacaftor/
ivacaftor
tezacaftor/ivacaftor↑ ivacaftor
↑ elexacaftor/tezacaftor/
ivacaftor
↑ tezacaftor/ivacaftorReduce dosage when co-administered with PAXLOVID. Refer to individual product labels for more information.
Dipeptidyl peptidase 4 (DPP4) inhibitors
saxagliptin
↑ saxagliptin
Dosage adjustment of saxagliptin is recommended. Refer to the saxagliptin product label for more information.
Endothelin receptor antagonists
bosentan
↑ bosentan
↓ nirmatrelvir/ritonavirDiscontinue use of bosentan at least 36 hours prior to initiation of PAXLOVID.
Refer to the bosentan product label for further information.Ergot derivatives
dihydroergotamine,
ergotamine,
methylergonovine↑ dihydroergotamine
↑ ergotamine
↑ methylergonovineCo-administration contraindicated due to potential for acute ergot toxicity characterized by vasospasm and ischemia of the extremities and other tissues including the central nervous system [see Contraindications (4)].
Hepatitis C direct acting antivirals
elbasvir/grazoprevir
↑ antiviral
Increased grazoprevir concentrations can result in alanine transaminase (ALT) elevations.
glecaprevir/pibrentasvir
Avoid concomitant use of glecaprevir/pibrentasvir with PAXLOVID.
ombitasvir/paritaprevir/ ritonavir and dasabuvir
Refer to the ombitasvir/paritaprevir/ritonavir and dasabuvir label for further information.
sofosbuvir/velpatasvir/ voxilaprevir
Refer to the sofosbuvir/velpatasvir/voxilaprevir product label for further information.
Patients on ritonavir-containing HCV regimens should continue their treatment as indicated. Monitor for increased PAXLOVID or HCV drug adverse events with concomitant use.Herbal products
St. John's Wort (hypericum perforatum)
↓ nirmatrelvir/ritonavir
Co-administration contraindicated due to potential loss of virologic response and possible resistance [see Contraindications (4)].
HMG-CoA reductase inhibitors
lovastatin,
simvastatin↑ lovastatin
↑ simvastatinCo-administration contraindicated due to potential for myopathy including rhabdomyolysis [see Contraindications (4)].
If treatment with PAXLOVID is considered medically necessary, discontinue use of lovastatin and simvastatin at least 12 hours prior to initiation of PAXLOVID, during the 5 days of PAXLOVID treatment, and for 5 days after completing PAXLOVID.HMG-CoA reductase inhibitors
atorvastatin
↑ atorvastatin
Consider temporary discontinuation of atorvastatin during treatment with PAXLOVID. Atorvastatin does not need to be withheld prior to or after completing PAXLOVID.
Hormonal contraceptive
ethinyl estradiol
↓ ethinyl estradiol
An additional, non-hormonal method of contraception should be considered during the 5 days of PAXLOVID treatment and until one menstrual cycle after stopping PAXLOVID.
Immunosuppressants
voclosporin
↑ voclosporin
Co-administration contraindicated due to potential for acute and/or chronic nephrotoxicity [see Contraindications (4)].
Immunosuppressants
calcineurin inhibitors:
cyclosporine,
tacrolimus
↑ cyclosporine
↑ tacrolimusAvoid concomitant use of calcineurin inhibitors with PAXLOVID when close monitoring of immunosuppressant concentrations is not feasible. If co-administered, dose adjustment of the immunosuppressant and close and regular monitoring for immunosuppressant concentrations and adverse reactions are recommended during and after treatment with PAXLOVID. Obtain expert consultation to appropriately manage the complexity of this co-administration [see Warnings and Precautions (5.1)].
mTOR inhibitors:
everolimus,
sirolimus
↑ everolimus
↑ sirolimus
Avoid concomitant use of everolimus and sirolimus and PAXLOVID.
Refer to the individual immunosuppressant product label and latest guidelines for further information.Janus kinase (JAK) inhibitors
tofacitinib
↑ tofacitinib
Dosage adjustment of tofacitinib is recommended. Refer to the tofacitinib product label for more information.
upadacitinib
↑ upadacitinib
Dosing recommendations for co-administration of upadacitinib with PAXLOVID depends on the upadacitinib indication. Refer to the upadacitinib product label for more information.
Long-acting beta-adrenoceptor agonist
salmeterol
↑ salmeterol
Avoid concomitant use with PAXLOVID. The combination may result in increased risk of cardiovascular adverse events associated with salmeterol, including QT prolongation, palpitations, and sinus tachycardia.
Microsomal triglyceride transfer protein (MTTP) inhibitor
lomitapide
↑ lomitapide
Co-administration contraindicated due to potential for hepatotoxicity and gastrointestinal adverse reactions [see Contraindications (4)].
Migraine medications
eletriptan
↑ eletriptan
Co-administration of eletriptan within at least 72 hours of PAXLOVID is contraindicated due to potential for serious adverse reactions including cardiovascular and cerebrovascular events [see Contraindications (4)].
ubrogepant
↑ ubrogepant
Co-administration of ubrogepant with PAXLOVID is contraindicated due to potential for serious adverse reactions [see Contraindications (4)].
Migraine medications
rimegepant
↑ rimegepant
Avoid concomitant use with PAXLOVID.
Mineralocorticoid receptor antagonists
finerenone
↑ finerenone
Co-administration contraindicated due to potential for serious adverse reactions including hyperkalemia, hypotension, and hyponatremia [see Contraindications (4)].
Muscarinic receptor antagonists
darifenacin
↑ darifenacin
The darifenacin daily-dose should not exceed 7.5 mg when co-administered with PAXLOVID. Refer to the darifenacin product label for more information.
Narcotic analgesics
fentanyl,
hydrocodone,
oxycodone,
meperidine↑ fentanyl
↑ hydrocodone
↑ oxycodone
↑ meperidineCareful monitoring of therapeutic and adverse effects (including potentially fatal respiratory depression) is recommended when fentanyl, hydrocodone, oxycodone, or meperidine is concomitantly administered with PAXLOVID. If concomitant use with PAXLOVID is necessary, consider a dosage reduction of the narcotic analgesic and monitor patients closely at frequent intervals. Refer to the individual product label for more information.
methadone
↓ methadone
Monitor methadone-maintained patients closely for evidence of withdrawal effects and adjust the methadone dose accordingly.
Neuropsychiatric agents
suvorexant
↑ suvorexant
Avoid concomitant use of suvorexant with PAXLOVID.
aripiprazole,
brexpiprazole,
cariprazine,
iloperidone,
lumateperone,
pimavanserin↑ aripiprazole
↑ brexpiprazole
↑ cariprazine
↑ iloperidone
↑ lumateperone
↑ pimavanserinDosage adjustment of aripiprazole, brexpiprazole, cariprazine, iloperidone, lumateperone, and pimavanserin is recommended. Refer to individual product label for more information.
Opioid antagonists
naloxegol
↑ naloxegol
Co-administration contraindicated due to the potential for opioid withdrawal symptoms [see Contraindications (4)].
Pulmonary hypertension agents (PDE5 inhibitors)
sildenafil (Revatio®)
↑ sildenafil
Co-administration of sildenafil with PAXLOVID is contraindicated for use in pulmonary hypertension due to the potential for sildenafil associated adverse events, including visual abnormalities hypotension, prolonged erection, and syncope [see Contraindications (4)].
Pulmonary hypertension agents (PDE5 inhibitors)
tadalafil (Adcirca®)
↑ tadalafil
Avoid concomitant use of tadalafil with PAXLOVID for pulmonary hypertension.
Pulmonary hypertension agents (sGC stimulators)
riociguat
↑ riociguat
Dosage adjustment is recommended for riociguat when used for pulmonary hypertension. Refer to the riociguat product label for more information.
Erectile dysfunction agents (PDE5 inhibitors)
avanafil
↑ avanafil
Do not use PAXLOVID with avanafil because a safe and effective avanafil dosage regimen has not been established.
sildenafil,
tadalafil,
vardenafil↑ sildenafil
↑ tadalafil
↑ vardenafilDosage adjustment is recommended for use of sildenafil, tadalafil or vardenafil with PAXLOVID when used for erectile dysfunction. Refer to individual product label for more information.
Sedative/hypnotics
triazolam,
oral midazolam*↑ triazolam
↑ midazolamCo-administration contraindicated due to potential for extreme sedation and respiratory depression [see Contraindications (4)].
Sedative/hypnotics
buspirone,
clorazepate,
diazepam,
estazolam,
flurazepam,
zolpidem↑ sedative/hypnotic
A dose decrease may be needed for these drugs when co-administered with PAXLOVID and monitoring for adverse events.
midazolam (administered parenterally)
↑ midazolam
Co-administration of midazolam (parenteral) should be done in a setting which ensures close clinical monitoring and appropriate medical management in case of respiratory depression and/or prolonged sedation. Dosage reduction for midazolam should be considered, especially if more than a single dose of midazolam is administered.
Refer to the midazolam product label for further information.Serotonin receptor 1A agonist/ serotonin receptor 2A antagonist
flibanserin
↑ flibanserin
Co-administration contraindicated due to potential for hypotension, syncope, and CNS depression [see Contraindications (4)].
Vasopressin receptor antagonists
tolvaptan
↑ tolvaptan
Co-administration contraindicated due to potential for dehydration, hypovolemia and hyperkalemia [see Contraindications (4)].
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data on the use of nirmatrelvir during pregnancy are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage, or ...
8.1 Pregnancy
Risk Summary
Available data on the use of nirmatrelvir during pregnancy are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Published observational studies on ritonavir use in pregnant women have not identified an increase in the risk of major birth defects. Published studies with ritonavir are insufficient to identify a drug associated risk of miscarriage (see Data). There are maternal and fetal risks associated with untreated COVID-19 in pregnancy (see Clinical Considerations).
In an embryo-fetal development study with nirmatrelvir, reduced fetal body weights following oral administration of nirmatrelvir to pregnant rabbits were observed at systemic exposures (AUC) approximately 11 times higher than clinical exposure at the approved human dose of PAXLOVID. No other adverse developmental outcomes were observed in animal reproduction studies with nirmatrelvir at systemic exposures (AUC) greater than or equal to 3 times higher than clinical exposure at the approved human dose of PAXLOVID (see Data).
In embryo-fetal developmental studies with ritonavir, no evidence of adverse developmental outcomes was observed following oral administration of ritonavir to pregnant rats and rabbits at systemic exposures (AUC) 5 (rat) or 8 (rabbits) times higher than clinical exposure at the approved human dose of PAXLOVID (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Human Data
Ritonavir
Based on prospective reports to the antiretroviral pregnancy registry of live births following exposure to ritonavir-containing regimens (including over 3,500 live births exposed in the first-trimester and over 3,500 live births exposed in the second and third trimesters), there was no difference in the rate of overall birth defects for ritonavir compared with the background birth defect rate of 2.7% in the U.S. reference population of the Metropolitan Atlanta Congenital Defects Program (MACDP). The prevalence of birth defects in live births was 2.4% [95% confidence interval (CI): 1.9%, 2.9%] following first-trimester exposure to ritonavir-containing regimens and 2.9% (95% CI: 2.4%, 3.5%) following second and third trimester exposure to ritonavir-containing regimens. While placental transfer of ritonavir and fetal ritonavir concentrations are generally low, detectable levels have been observed in cord blood samples and neonate hair.
Animal Data
Nirmatrelvir
Embryo-fetal developmental (EFD) toxicity studies were conducted in pregnant rats and rabbits administered oral nirmatrelvir doses of up to 1,000 mg/kg/day during organogenesis [on Gestation Days (GD) 6 through 17 in rats and GD 7 through 19 in rabbits]. No biologically significant developmental effects were observed in the rat EFD study. At the highest dose of 1,000 mg/kg/day, the systemic nirmatrelvir exposure (AUC24) in rats was approximately 9 times higher than clinical exposures at the approved human dose of PAXLOVID. In the rabbit EFD study, lower fetal body weights (9% decrease) were observed at 1,000 mg/kg/day in the absence of significant maternal toxicity findings. At 1,000 mg/kg/day, the systemic exposure (AUC24) in rabbits was approximately 11 times higher than clinical exposures at the approved human dose of PAXLOVID. No other significant developmental toxicities (malformations and embryo-fetal lethality) were observed up to the highest dose tested, 1,000 mg/kg/day. No developmental effects were observed in rabbits at 300 mg/kg/day resulting in systemic exposure (AUC24) approximately 3 times higher than clinical exposures at the approved human dose of PAXLOVID. A pre- and postnatal developmental (PPND) study in pregnant rats administered oral nirmatrelvir doses of up to 1,000 mg/kg/day from GD 6 through Lactation Day (LD) 20 showed no adverse findings. Although no difference in body weight was noted at birth when comparing offspring born to nirmatrelvir-treated versus control animals, a decrease in the body weight of offspring was observed on Postnatal Day (PND) 17 (8% decrease) and PND 21 (up to 7% decrease) in the absence of maternal toxicity. No significant differences in offspring body weight were observed from PND 28 to PND 56. The maternal systemic exposure (AUC24) at 1,000 mg/kg/day was approximately 9 times higher than clinical exposures at the approved human dose of PAXLOVID. No body weight changes in the offspring were noted at 300 mg/kg/day, where maternal systemic exposure (AUC24) was approximately 6 times higher than clinical exposures at the approved human dose of PAXLOVID.
Ritonavir
Ritonavir was administered orally to pregnant rats (at 0, 15, 35, and 75 mg/kg/day) and rabbits (at 0, 25, 50, and 110 mg/kg/day) during organogenesis (on GD 6 through 17 in rats and GD 6 through 19 in rabbits). No evidence of teratogenicity due to ritonavir was observed in rats and rabbits at systemic exposures (AUC) 5 (rats) or 8 (rabbits) times higher than exposure at the approved human dose of PAXLOVID. Increased incidences of early resorptions, ossification delays, and developmental variations, as well as decreased fetal body weights were observed in rats in the presence of maternal toxicity, at systemic exposures (AUC) approximately 10 times higher than exposure at the approved human dose of PAXLOVID. In rabbits, resorptions, decreased litter size, and decreased fetal weights were observed at maternally toxic doses, at systemic exposures greater than 8 times higher than exposure at the approved human dose of PAXLOVID. In a PPND study in rats, administration of 0, 15, 35, and 60 mg/kg/day ritonavir from GD 6 through PND 20 resulted in no developmental toxicity, at ritonavir systemic exposures greater than 10 times the exposure at the approved human dose of PAXLOVID.
8.2 Lactation
Risk Summary
Nirmatrelvir and ritonavir are present in human breast milk in small amounts (less than 2%). In a clinical lactation study in 8 lactating women, nirmatrelvir and ritonavir were estimated to be present in human milk at a mean weight-normalized infant daily dose of 0.16 mg/kg/day (1.8% of maternal weight-adjusted daily dose) and 0.006 mg/kg/day (0.2% of maternal weight-adjusted daily dose), respectively (see Data).
There are no available data on the effects of nirmatrelvir or ritonavir on the breastfed infant or on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for PAXLOVID and any potential adverse effects on the breastfed infant from PAXLOVID or from the underlying maternal condition. Breastfeeding individuals with COVID-19 should follow practices according to clinical guidelines to avoid exposing the infant to COVID-19.
In a clinical pharmacokinetics study, 8 healthy lactating women who were at least 12 weeks postpartum were administered 3 oral doses every 12 hours (steady state dosing) of 300 mg/100 mg nirmatrelvir/ritonavir. The mean daily amount of nirmatrelvir and ritonavir recovered in breast milk was 0.752 mg and 0.027 mg, respectively, representing 0.13% and 0.014% of the corresponding administered daily maternal doses (unadjusted for weight). The estimated daily infant dose (assuming average milk consumption of 150 mL/kg/day), was 0.16 mg/kg/day and 0.006 mg/kg/day, 1.8% and 0.2% of the maternal dose, respectively, for nirmatrelvir and ritonavir.
8.3 Females and Males of Reproductive Potential
Contraception
Use of ritonavir may reduce the efficacy of combined hormonal contraceptives. Advise patients using combined hormonal contraceptives to use an effective alternative contraceptive method or an additional barrier method of contraception [see Drug Interactions (7.3)].
8.5 Geriatric Use
Clinical studies of PAXLOVID include subjects 65 years of age and older and their data contributes to the overall assessment of safety and efficacy [see Adverse Reactions (6.1) and Clinical Studies (14.1)]. Of the total number of subjects in the integrated dataset consisting of EPIC-HR and EPIC-SR who were randomized to and received PAXLOVID (N=1,578), 165 (10%) were 65 years of age and older and 39 (2%) were 75 years of age and older. No overall differences in safety were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in safety between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
Renal impairment increases nirmatrelvir exposure, which may increase the risk of PAXLOVID adverse reactions. No dosage adjustment is recommended in patients with mild renal impairment (eGFR ≥60 to <90 mL/min). Reduce the PAXLOVID dosage in patients with moderate renal impairment (eGFR ≥30 to <60 mL/min). Reduce the PAXLOVID dose and dose frequency in patients with severe renal impairment (eGFR <30 mL/min), including those requiring hemodialysis. On days when patients undergo hemodialysis, the PAXLOVID dose should be administered after hemodialysis [see Dosage and Administration (2.3), Adverse Reactions (6.1), and Clinical Pharmacology (12.3)]. Prescriptions should specify the numeric dose of each active ingredient within PAXLOVID. Providers should counsel patients about renal dosing instructions [see Patient Counseling Information (17)].
Close8.7 Hepatic Impairment
No dosage adjustment of PAXLOVID is recommended for patients with mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment. No pharmacokinetic or safety data are available regarding the use of nirmatrelvir or ritonavir in subjects with severe (Child-Pugh Class C) hepatic impairment, therefore, PAXLOVID is not recommended for use in patients with severe (Child-Pugh Class C) hepatic impairment [see Warnings and Precautions (5.3) and Clinical Pharmacology (12.3)].
-
10 OVERDOSAGETreatment of overdose with PAXLOVID should consist of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient. There is no specific ...
Treatment of overdose with PAXLOVID should consist of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient. There is no specific antidote for overdose with PAXLOVID.
Close -
11 DESCRIPTIONPAXLOVID is nirmatrelvir tablets co-packaged with ritonavir tablets. Nirmatrelvir is a SARS-CoV-2 main protease (Mpro) inhibitor, and ritonavir is an HIV-1 protease inhibitor and CYP3A inhibitor ...
PAXLOVID is nirmatrelvir tablets co-packaged with ritonavir tablets. Nirmatrelvir is a SARS-CoV-2 main protease (Mpro) inhibitor, and ritonavir is an HIV-1 protease inhibitor and CYP3A inhibitor.
Nirmatrelvir
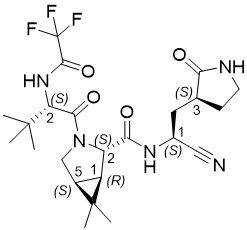
The chemical name of active ingredient of nirmatrelvir is (1R,2S,5S)-N-((1S)-1-Cyano-2-((3S)-2-oxopyrrolidin-3-yl)ethyl)-3-((2S)-3,3-dimethyl-2-(2,2,2-trifluoroacetamido)butanoyl)-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxamide]. It has a molecular formula of C23H32F3N5O4 and a molecular weight of 499.54. Nirmatrelvir has the following structural formula:
Nirmatrelvir is available as immediate-release, film-coated tablets. Each tablet contains 150 mg nirmatrelvir with the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, lactose monohydrate, microcrystalline cellulose, and sodium stearyl fumarate. The following are the ingredients in the film coating: hydroxy propyl methylcellulose, iron oxide red, polyethylene glycol, and titanium dioxide.
CloseRitonavir
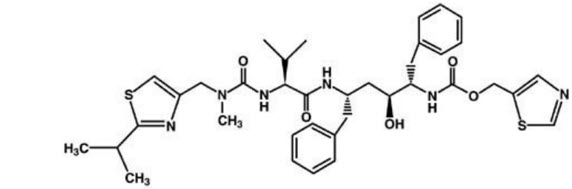
Ritonavir is chemically designated as 10-Hydroxy-2-methyl-5-(1-methylethyl)-1- [2-(1 methylethyl)-4-thiazolyl]-3,6-dioxo-8,11-bis(phenylmethyl)-2,4,7,12- tetraazatridecan-13-oic acid, 5-thiazolylmethyl ester, [5S-(5R*,8R*,10R*,11R*)]. Its molecular formula is C37H48N6O5S2, and its molecular weight is 720.95. Ritonavir has the following structural formula:
Ritonavir is available as film-coated tablets. Each tablet contains 100 mg ritonavir with the following inactive ingredients: anhydrous dibasic calcium phosphate, colloidal silicon dioxide, copovidone, sodium stearyl fumarate, and sorbitan monolaurate. The film coating may include the following ingredients: colloidal anhydrous silica, colloidal silicon dioxide, hydroxypropyl cellulose, hypromellose, polyethylene glycol, polysorbate 80, talc, and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Nirmatrelvir is a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antiviral drug [see Microbiology (12.4)]. Ritonavir is an HIV-1 protease inhibitor but ...
12.1 Mechanism of Action
Nirmatrelvir is a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antiviral drug [see Microbiology (12.4)].
Ritonavir is an HIV-1 protease inhibitor but is not active against SARS-CoV-2 Mpro. Ritonavir inhibits the CYP3A-mediated metabolism of nirmatrelvir, resulting in increased plasma concentrations of nirmatrelvir.
12.2 Pharmacodynamics
Cardiac Electrophysiology
At 3 times the steady state peak plasma concentration (Cmax) at the recommended dose, nirmatrelvir does not prolong the QTc interval to any clinically relevant extent.
12.3 Pharmacokinetics
The pharmacokinetics of nirmatrelvir/ritonavir were similar in healthy subjects and in subjects with mild-to-moderate COVID-19.
Nirmatrelvir AUC increased in a less than dose proportional manner over a single dose range from 250 mg to 750 mg (0.83 to 2.5 times the approved recommended dose) and multiple dose range from 75 mg to 500 mg (0.25 to 1.67 times the approved recommended dose), when administered in combination with 100 mg ritonavir. Nirmatrelvir steady state was achieved on Day 2 following administration of the approved recommended dosage and the mean accumulation ratio was approximately 2-fold.
The pharmacokinetic properties of nirmatrelvir/ritonavir are displayed in Table 3.
Table 3: Pharmacokinetic Properties of Nirmatrelvir and Ritonavir in Healthy Subjects Nirmatrelvir (When Given With Ritonavir) Ritonavir Abbreviations: CL/F=apparent clearance; hr=hour; L/hr=liters per hour; T½=terminal elimination half-life; Tmax=the time to reach Cmax; Vz/F=apparent volume of distribution. - *
- Represents data after a single dose of 300 mg nirmatrelvir (2 × 150 mg tablet formulation) administered together with 100 mg ritonavir tablet in healthy subjects.
- †
- Following a single oral dose of nirmatrelvir 300 mg boosted ritonavir 100 mg at -12 hours, 0 hours and 12 hours, administered under fed (high fat and high calorie meal) or fasted conditions.
- ‡
- Red blood cell to plasma ratio.
- §
- 300 mg nirmatrelvir (oral suspension formulation) co-administered with 100 mg ritonavir (tablet formulation) twice daily for 3 days.
- ¶
- Determined by 19F-NMR analysis following 300 mg nirmatrelvir oral suspension administered at 0 hr enhanced with 100 mg ritonavir at -12 hours, 0 hours, 12 hours, and 24 hours.
- #
- Determined by 14C analysis following 600 mg 14C-ritonavir oral solution (6 times the approved ritonavir dose).
Absorption
Tmax (hr), median
3.00*
3.98*
Food effect
Test/reference (fed/fasted) ratios of adjusted geometric means (90% CI) AUCinf and Cmax for nirmatrelvir were 119.67 (108.75, 131.68) and 161.01 (139.05, 186.44), respectively.†
Distribution
% bound to human plasma proteins
69%
98–99%
Blood-to-plasma ratio
0.60
0.14‡
Vz/F (L), mean
104.7§
112.4§
Elimination
Major route of elimination
Renal elimination
Hepatic metabolism
Half-life (T½) (hr), mean
6.05*
6.15*
Oral clearance (CL/F) (L/hr), mean
8.99§
13.92§
Metabolism
Metabolic pathways
Nirmatrelvir is a CYP3A substrate but when dosed with ritonavir, metabolic clearance is minimal.
Major CYP3A, Minor CYP2D6
Excretion
% drug-related material in feces
35.3%¶
86.4%#
% of dose excreted as total (unchanged drug) in feces
27.5%¶
33.8%#
% drug-related material in urine
49.6%¶
11.3%#
% of dose excreted as total (unchanged drug) in urine
55.0%¶
3.5%#
The predicted Day 5 nirmatrelvir exposure parameters in adult subjects with mild-to-moderate COVID-19 who were treated with PAXLOVID in EPIC-HR are presented in Table 4.
Table 4: Predicted Day 5 Nirmatrelvir Exposure Parameters Following Administration of Nirmatrelvir/Ritonavir 300 mg/100 mg Twice Daily in Subjects with Mild-to-Moderate COVID-19 Pharmacokinetic Parameter (units)* Nirmatrelvir† Abbreviations: Cmax=predicted maximal concentration; Cmin=predicted minimal concentration (Ctrough). Cmax (µg/mL)
3.29 (1.93, 5.40)
AUCtau (µg*hr/mL)‡
28.3 (12.5, 52.5)
Cmin (µg/mL)
1.40 (0.48, 3.45)
Effect of Food
No clinically significant differences in the pharmacokinetics of nirmatrelvir were observed following administration of a high fat meal (800-1,000 calories; 50% fat) to healthy subjects.
Specific Populations
There were no clinically significant differences in the pharmacokinetics of nirmatrelvir based on age (18 to 86 years), sex, or race/ethnicity.
Pediatric Patients
The pharmacokinetics of nirmatrelvir/ritonavir in patients less than 18 years of age have not been established.
Patients with Renal Impairment
The pharmacokinetics of nirmatrelvir in subjects with renal impairment following administration of a single oral dose of nirmatrelvir 100 mg (0.33 times the approved recommended dose) co-administered with ritonavir 100 mg were determined. Compared to healthy controls with no renal impairment, the Cmax and AUC of nirmatrelvir in subjects with mild renal impairment was 30% and 24% higher, in subjects with moderate renal impairment was 38% and 87% higher, and in subjects with severe renal impairment was 48% and 204% higher, respectively.
The pharmacokinetics of nirmatrelvir in subjects with mild-to-moderate COVID-19 and severe renal impairment (eGFR<30 mL/min) either requiring intermittent hemodialysis (n=12) or not requiring hemodialysis (n=2) were evaluated after administration of 300 mg/100 mg nirmatrelvir/ritonavir once on Day 1 followed by 150 mg/100 mg nirmatrelvir/ritonavir once daily on Days 2-5 for a total of 5 doses.
The administration of 300 mg/100 mg nirmatrelvir/ritonavir once on Day 1 followed by 150 mg/100 mg nirmatrelvir/ritonavir once daily on Days 2-5 in subjects with severe renal impairment, either requiring intermittent hemodialysis or not requiring hemodialysis resulted in comparable exposures on Day 1 and at steady-state (AUC0-24 and Cmax) compared to those observed in subjects with normal renal function receiving 300 mg/100 mg nirmatrelvir/ritonavir twice daily for 5 days. During a 4-hour hemodialysis session, approximately 6.9% of nirmatrelvir dose was cleared through dialysis. Hemodialysis clearance was 1.83 L/h.
Patients with Hepatic Impairment
The pharmacokinetics of nirmatrelvir were similar in patients with moderate (Child-Pugh Class B) hepatic impairment compared to healthy subjects following administration of a single oral dose of nirmatrelvir 100 mg (0.33 times the approved recommended dose) co-administered with ritonavir 100 mg. The impact of severe hepatic impairment (Child-Pugh Class C) on the pharmacokinetics of nirmatrelvir or ritonavir has not been studied.
Clinical Drug Interaction Studies
Table 5 describes the effect of other drugs on the Cmax and AUC of nirmatrelvir.
Table 5: The Effect of Other Drugs on the Pharmacokinetic Parameters of Nirmatrelvir Co-administered Drug Dose (Schedule) N Percent Ratio (in combination with co-administered drug/alone) of Nirmatrelvir Pharmacokinetic Parameters (90% CI);
No Effect=100Co-administered Drug Nirmatrelvir/ Ritonavir Cmax AUC* Abbreviations: AUC=area under the plasma concentration-time curve; AUCinf=area under the plasma concentration-time profile from time zero extrapolated to infinite time; AUCtau=area under the plasma concentration-time profile from time zero to time tau (τ), the dosing interval. CI=confidence interval; Cmax=observed maximum plasma concentrations. Carbamazepine†
300 mg twice daily
(16 doses)300 mg/100 mg once daily
(2 doses)10
56.82
(47.04, 68.62)44.50
(33.77, 58.65)Itraconazole
200 mg once daily
(8 doses)300 mg/100 mg twice daily
(5 doses)11
118.57
(112.50, 124.97)138.82
(129.25, 149.11)Table 6 describes the effect of nirmatrelvir/ritonavir on the Cmax and AUCinf of other drugs.
Table 6: Effect of Nirmatrelvir/Ritonavir on Pharmacokinetics of Other Drugs Co-administered Drug Dose (Schedule) N Percent Ratio of Test/Reference of Geometric Means (90% CI);
No Effect=100Co-administered Drug Nirmatrelvir/ Ritonavir Cmax AUCinf Abbreviations: AUCinf=area under the plasma concentration-time curve from time zero extrapolated to infinite time; CI=confidence interval; Cmax=observed maximum plasma concentrations; CYP3A4=cytochrome P450 3A4; OATP1B1=organic anion transporter polypeptide 1B1; P-gp=p-glycoprotein. - *
- For midazolam, Test=nirmatrelvir/ritonavir plus midazolam, Reference=Midazolam. Midazolam is an index substrate for CYP3A4. For dabigatran, Test=nirmatrelvir/ritonavir plus dabigatran, Reference=Dabigatran. Dabigatran is an index substrate for P-gp. For rosuvastatin, Test=nirmatrelvir/ritonavir plus rosuvastatin, Reference=Rosuvastatin. Rosuvastatin is an index substrate for OATP1B1.
Midazolam*
2 mg
(1 dose)300 mg/100 mg twice daily
(9 doses)10
368.33
(318.91, 425.41)1430.02
(1204.54, 1697.71)Dabigatran*
75 mg
(1 dose)300 mg/100 mg twice daily
(4 doses)24
233.06
(172.14, 315.54)194.47
(155.29, 243.55)Rosuvastatin*
10 mg
(1 dose)
300 mg/100 mg twice daily
(3 doses)12
212.44
(174.31, 258.90)
131.18
(115.89, 148.48)
In Vitro Studies
Cytochrome P450 (CYP) Enzymes:
- •
- Nirmatrelvir is a reversible and time-dependent inhibitor of CYP3A, but not an inhibitor CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, or CYP2D6. Nirmatrelvir is an inducer of CYP2B6, 2C8, 2C9, and 3A4, but there is minimal risk for pharmacokinetic interactions arising from induction of these CYP enzymes at the proposed therapeutic dose.
- •
- Ritonavir is a substrate of CYP2D6 and CYP3A. Ritonavir is an inducer of CYP1A2, CYP2C9, CYP2C19, CYP2B6, and CYP3A.
Transporter Systems: Nirmatrelvir is an inhibitor of P-gp and OATP1B1. Nirmatrelvir is a substrate for P-gp, but not BCRP, MATE1, MATE2K, NTCP, OAT1, OAT2, OAT3, OCT1, OCT2, PEPT1, OATP1B1, OATP1B3, OATP2B1, or OATP4C1.
Close12.4 Microbiology
Mechanism of Action
Nirmatrelvir is a peptidomimetic inhibitor of the SARS-CoV-2 main protease (Mpro), also referred to as 3C-like protease (3CLpro) or nonstructural protein 5 (nsp5) protease. Inhibition of SARS-CoV-2 Mpro renders it incapable of processing the viral polyproteins pp1a and pp1ab, preventing viral replication. Nirmatrelvir inhibited the activity of recombinant SARS-CoV-2 Mpro in a biochemical assay with a Ki value of 3.1 nM and an IC50 value of 19.2 nM. Nirmatrelvir was found to bind directly to the SARS-CoV-2 Mpro active site by X-ray crystallography.
Antiviral Activity
Cell Culture Antiviral Activity
Nirmatrelvir exhibited antiviral activity against SARS-CoV-2 (USA-WA1/2020 isolate) infection of differentiated normal human bronchial epithelial (dNHBE) cells with EC50 and EC90 values of 62 nM (31 ng/mL) and 181 nM (90 ng/mL), respectively, after 3 days of drug exposure.
The antiviral activity of nirmatrelvir against the Omicron sub-variants BA.2, BA.2.12.1, BA.4, BA.4.6, BA.5, BF.7, BQ.1, BQ.1.11, XBB.1.5, EG.5, and JN.1 was assessed in Vero E6-TMPRSS2 cells in the presence of a P-gp inhibitor. Nirmatrelvir had a median EC50 value of 88 nM (range: 39-146 nM) against the Omicron sub-variants, reflecting EC50 value fold changes ≤1.8 relative to the USA-WA1/2020 isolate.
In addition, the antiviral activity of nirmatrelvir against the SARS-CoV-2 Alpha, Beta, Gamma, Delta, Lambda, Mu, and Omicron BA.1 variants was assessed in Vero E6 P-gp knockout cells. Nirmatrelvir had a median EC50 value of 25 nM (range: 16-141 nM). The Beta variant was the least susceptible variant tested, with an EC50 value fold change of 3.7 relative to USA-WA1/2020. The other variants had EC50 value fold changes ≤1.1 relative to USA-WA1/2020.
Clinical Antiviral Activity
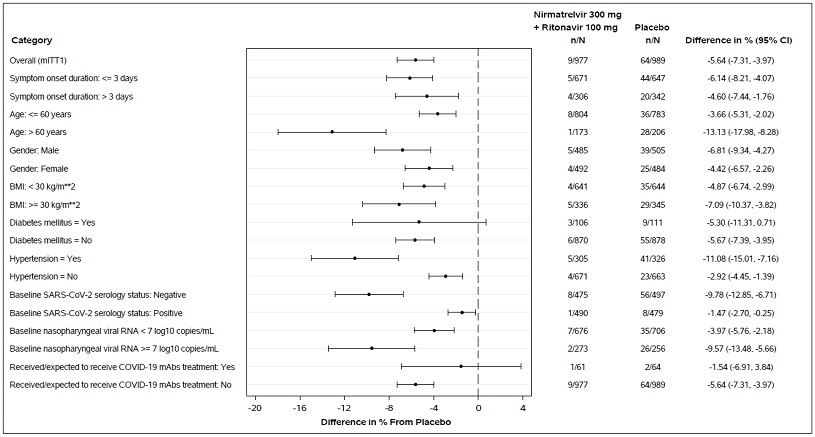
In clinical trial EPIC-HR, which enrolled subjects who were primarily infected with the SARS-CoV-2 Delta variant, PAXLOVID treatment was associated with a 0.83 log10 copies/mL greater median decline in viral RNA shedding levels in nasopharyngeal samples through Day 5 (mITT1 analysis set, all treated subjects with onset of symptoms ≤5 days who at baseline did not receive nor were expected to receive COVID-19 therapeutic mAb treatment); similar results were observed in the mITT2 analysis set (all treated subjects with onset of symptoms ≤5 days). In the EPIC-SR trial, which included subjects who were infected with SARS-CoV-2 Delta (79%) or Omicron (19%) variants, PAXLOVID treatment was associated with a 1.05 log10 copies/mL greater median decline in viral RNA shedding levels in nasopharyngeal samples through Day 5, with similar declines observed in subjects infected with Delta or Omicron variants. The degree of reduction in viral RNA levels relative to placebo following 5 days of PAXLOVID treatment was similar between unvaccinated high-risk subjects in EPIC-HR and vaccinated high-risk subjects in EPIC-SR.
Antiviral Resistance
In Cell Culture and Biochemical Assays
SARS-CoV-2 Mpro residues potentially associated with nirmatrelvir resistance have been identified using a variety of methods, including SARS-CoV-2 resistance selection, testing of recombinant SARS-CoV-2 viruses with Mpro substitutions, and biochemical assays with recombinant SARS-CoV-2 Mpro containing amino acid substitutions. Table 7 indicates Mpro substitutions and combinations of Mpro substitutions that have been observed in SARS-CoV-2 under nirmatrelvir selective pressure in cell culture. Individual Mpro substitutions are listed regardless of whether they occurred alone or in combination with other Mpro substitutions. Note that the Mpro S301P and T304I substitutions overlap the P6 and P3 positions of the nsp5/nsp6 cleavage site located at the C-terminus of Mpro. Substitutions at other Mpro cleavage sites have not been associated with nirmatrelvir resistance in cell culture. The clinical significance of these substitutions is unknown.
Abbreviation: ND=no data. - *
- EC50 value fold change ranges are shown in instances where multiple data points have been reported.
Table 7: SARS-CoV-2 Mpro Amino Acid Substitutions Selected by Nirmatrelvir in Cell Culture*
Single Substitutions
(EC50 value fold change in cell culture)
T21I (1.1-4.8), S46F (ND), L50F (1.2-4.2), P108S (ND), T135I (ND), F140L (4.1), S144A (2.2-5.3), C160F (2.1), E166A (3.3), E166V (25‑288), L167F (1.9-2.5), T169I (ND), H172Y (15), A173V (0.9-2.3), V186A (ND), R188G (ND), A191V (0.7-1.5), A193P (ND), P252L (5.9), S301P (ND), and T304I (1.4-5.5).
≥2 Substitutions
(EC50 value fold change in cell culture)
T21I+S144A (9.4), T21I+E166V (83-250), T21I+A173V (3.1-8.9), T21I+T304I (3.0-7.9), L50F+E166V (34-175), L50F+T304I (5.9), T135I+T304I (3.8), F140L+A173V (10-17), H172Y+P252L (ND), A173V+T304I (5.8-20), T21I+L50F+A193P+S301P (29), T21I+S144A+T304I (11-28), T21I+C160F+A173V+V186A+T304I (28-29), T21I+A173V+T304I (15-16), and L50F+F140L+L167F+T304I (43-55).
Table 8 indicates Mpro substitutions and combinations of Mpro substitutions that have been found to reduce nirmatrelvir activity ≥3-fold (based on IC50 or Ki values) in biochemical assays using recombinant SARS-CoV-2 Mpro. Note that these Mpro substitutions were laboratory engineered and most were not observed in PAXLOVID-treated subjects in clinical trials. In addition, according to public sequence databases, most of these substitutions have not been observed in clinical isolates or have been observed but with global cumulative frequencies ≤0.002%. Thus, the clinical relevance of these substitutions is unclear. The following Mpro substitutions and combinations of Mpro substitutions emerged in cell culture in the presence of nirmatrelvir but conferred <3-fold reduced nirmatrelvir activity in biochemical assays: T21I, S46F, L50F, P108S, T135I, C160F, T169I, V186A, A191V, A193P, P252L, S301P, T304I, T21I+T304I, and L50F+T304I.
Table 8: SARS-CoV-2 Mpro Amino Acid Substitutions That Reduce Nirmatrelvir Activity ≥3-Fold in Biochemical Assays
Single Substitutions
(IC50/Ki value fold change in biochemical assay)
Y54A/C (3.0-25), F140A/L/S (1.2-230), G143S (3.6-148), S144A/F/G/M/W/Y (1.2-76), S144D/E/H/Q/T/V (81-480), S144K/L/P/R (1,165->5,319), H164N (1.9-6.7), M165D/F/G/T (5.7-51), M165H/K/P/R/W (>384), M165Y (3,838), E166A/G/K/L/Q (4.5-77), E166D/H/I/N/V/Y (143-708), E166R/V (>1,538-7,700), L167F (1.4-4.5), P168del (4.5-9.3), H172D/F/G/K/Q/Y (10-91), H172A/C/E/M/N/R/V/Y (114-858), H172I/L/S/T (1,172-6,740), A173S/V (4.1-52), R188G (38), Q189E/K (1.6-16), Q192A/C/D/E/F/G/H/I/K/L/P/R/S/T/V/W (5.0-61), Q192Y (>384), A260V (0.6-3.3), and V297A (3.0).
≥2 Substitutions
(IC50/Ki value fold change in biochemical assay)
T21I+S144A (20), T21I+E166V (120-11,000), T21I+A173V (15), L50F+E166V (100-4,500), T135I+T304I (5.1), F140L+A173V (95), S144A+T304I (28), E166V+L232R (5,700), P168del+A173V (170-536), H172Y+P252L (180), A173V+T304I (28), T21I+S144A+T304I (51), T21I+A173V+T304I (55), L50F+E166A+L167F (52-180), T21I+L50F+A193P+S301P (7.3), L50F+F140L+L167F+T304I (190), and T21I+C160F+A173V+V186A+T304I (28).
In Clinical Trials
Treatment-emergent substitutions were evaluated among subjects in clinical trials EPIC-HR/SR with sequence data available at both baseline and post-baseline visits (n=907 PAXLOVID-treated subjects, n=946 placebo-treated subjects). SARS-CoV-2 Mpro amino acid changes were classified as PAXLOVID treatment-emergent substitutions if they occurred at the same amino acid position in 3 or more PAXLOVID-treated subjects and were ≥2.5-fold more common in PAXLOVID-treated subjects than placebo-treated subjects. The following PAXLOVID treatment-emergent Mpro substitutions were observed: T98I/R/del(n=4), E166V (n=3), and W207L/R/del (n=4). In biochemical assays, the T98I and W207L/R substitutions did not affect nirmatrelvir activity (Ki value fold changes were 0.3 and 0.7/0.3, respectively), whereas the E166V substitution (which occurs at a Mpro-nirmatrelvir contact residue) reduced nirmatrelvir activity 187-7,700-fold. Within the Mpro cleavage sites, the following PAXLOVID treatment-emergent substitutions were observed: A5328S/V(n=7) and S6799A/P/Y (n=4). These cleavage site substitutions were not associated with the co-occurrence of any specific Mpro substitutions. In a cell culture replicon assay, the A5328S/V and S6799A substitutions did not affect nirmatrelvir activity (EC50 value fold changes were 0.3/0.2 and 0.7, respectively).
None of the treatment-emergent substitutions listed above in Mpro or Mpro cleavage sites occurred in PAXLOVID-treated subjects who experienced hospitalization. Thus, the clinical significance of these substitutions is unknown.
Viral RNA Rebound and Treatment-Emergent Substitutions
EPIC-HR and EPIC-SR were not designed to evaluate COVID-19 rebound; exploratory analyses were conducted to assess the relationship between PAXLOVID use and rebound in viral RNA shedding levels.
Post-treatment increases in SARS-CoV-2 RNA shedding levels in nasopharyngeal samples were observed on Day 10 and/or Day 14 in a subset of PAXLOVID and placebo recipients in EPIC-HR and EPIC-SR, irrespective of COVID-19 symptoms. The frequency of detection of post-treatment viral RNA rebound varied according to analysis parameters, but was generally similar among PAXLOVID and placebo recipients. A similar or smaller percentage of placebo recipients compared to PAXLOVID recipients had nasopharyngeal viral RNA results < lower limit of quantitation (LLOQ) at all study timepoints in both the treatment and post-treatment periods.
In EPIC-HR, of 59 PAXLOVID-treated subjects identified with post-treatment viral RNA rebound and with available viral sequence data, treatment-emergent substitutions in Mpro potentially reducing nirmatrelvir activity were detected in 2 (3%) subjects, including E166V in 1 subject and T304I in 1 subject. Both subjects had viral RNA shedding levels <LLOQ by Day 14.
Post-treatment viral RNA rebound was not associated with the primary clinical outcome of COVID-19 related hospitalization or death from any cause through Day 28 following the single 5-day course of PAXLOVID treatment. The clinical relevance of post-treatment increases in viral RNA following PAXLOVID or placebo treatment is unknown.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Nirmatrelvir - Carcinogenicity studies have not been conducted with nirmatrelvir. Nirmatrelvir was negative for mutagenic or ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Nirmatrelvir
Carcinogenicity studies have not been conducted with nirmatrelvir.
Nirmatrelvir was negative for mutagenic or clastogenic activity in a battery of in vitro and in vivo assays including the Ames bacterial reverse mutation assay using S. typhimurium and E. coli, the in vitro micronucleus assay using human lymphoblastoid TK6 cells, and the in vivo rat micronucleus assays.
In a fertility and early embryonic development study, nirmatrelvir was administered orally to male and female rats at doses of 60, 200, or 1,000 mg/kg/day once daily beginning 14 days prior to mating, throughout the mating phase, and continued through GD 6 for females and for a total of 32 doses for males. There were no effects on fertility, reproductive performance, or early embryonic development at doses up to 1,000 mg/kg/day, resulting in systemic exposure (AUC24) approximately 5 times higher than exposure at the approved human dose of PAXLOVID.
Ritonavir
Carcinogenicity studies in mice and rats have been conducted on ritonavir. In male mice, at levels of 50, 100, or 200 mg/kg/day, there was a dose dependent increase in the incidence of both adenomas and combined adenomas and carcinomas in the liver. Based on AUC measurements, the exposure at the high dose was approximately 25 times higher than the exposure in humans at the approved human dose of PAXLOVID. No carcinogenic effects were observed in females at up to the highest dose tested, resulting in systemic exposure (AUC24) approximately 25 times higher than the exposure in humans at the approved human dose of PAXLOVID. In rats dosed at levels of 7, 15, or 30 mg/kg/day, there were no carcinogenic effects. In this study, the exposure at the high dose was approximately 5 times higher than the exposure in humans at the approved human dose of PAXLOVID.
Ritonavir was found to be negative for mutagenic or clastogenic activity in a battery of in vitro and in vivo assays including the Ames bacterial reverse mutation assay using S. typhimurium and E. coli, the mouse lymphoma assay, the mouse micronucleus test and chromosomal aberration assays in human lymphocytes.
Ritonavir produced no effects on fertility in rats at drug exposures approximately 18 (male) and 27 (female) times higher than the exposure in humans at the approved human dose of PAXLOVID.
-
14 CLINICAL STUDIES14.1 Efficacy in Subjects at High Risk of Progression to Severe COVID-19 (EPIC-HR) EPIC-HR (NCT04960202) was a Phase 2/3, randomized, double-blind, placebo-controlled trial in non-hospitalized ...
14.1 Efficacy in Subjects at High Risk of Progression to Severe COVID-19 (EPIC-HR)
EPIC-HR (NCT04960202) was a Phase 2/3, randomized, double-blind, placebo-controlled trial in non-hospitalized symptomatic adult subjects with a laboratory confirmed diagnosis of SARS-CoV-2 infection. Eligible subjects were 18 years of age and older with at least 1 of the following risk factors for progression to severe disease: diabetes, overweight (BMI >25), chronic lung disease (including asthma), chronic kidney disease, current smoker, immunosuppressive disease or immunosuppressive treatment, cardiovascular disease, hypertension, sickle cell disease, neurodevelopmental disorders, active cancer, medically-related technological dependence, or were 60 years of age and older regardless of comorbidities. Subjects with COVID-19 symptom onset of ≤5 days were included in the study. Subjects were randomized (1:1) to receive PAXLOVID (nirmatrelvir/ritonavir 300 mg/100 mg) or placebo orally every 12 hours for 5 days. The trial excluded individuals with a history of prior COVID-19 infection or vaccination and excluded individuals taking any medications with clinically significant drug interactions with PAXLOVID. The primary efficacy endpoint was the proportion of subjects with COVID-19 related hospitalization or death from any cause through Day 28. The analysis was conducted in the modified intent-to-treat (mITT) analysis set [all treated subjects with onset of symptoms ≤3 days who at baseline did not receive nor were expected to receive COVID-19 therapeutic monoclonal antibody (mAb) treatment], the mITT1 analysis set (all treated subjects with onset of symptoms ≤5 days who at baseline did not receive nor were expected to receive COVID-19 therapeutic mAb treatment), and the mITT2 analysis set (all treated subjects with onset of symptoms ≤5 days).
A total of 2,113 subjects were randomized to receive either PAXLOVID or placebo. At baseline, mean age was 45 years; 51% were male; 71% were White, 15% were Asian, 9% were American Indian or Alaska Native, 4% were Black or African American, and 1% was missing or unknown; 41% were Hispanic or Latino; 67% of subjects had onset of symptoms ≤3 days before initiation of study treatment; 49% of subjects were serological negative at baseline; the mean (SD) baseline viral RNA in nasopharyngeal samples was 4.71 log10 copies/mL (2.89); 27% of subjects had a baseline viral RNA of ≥10^7 (log10 copies/mL); 6% of subjects either received or were expected to receive COVID-19 therapeutic monoclonal antibody treatment at the time of randomization and were excluded from the mITT and mITT1 analyses.
The baseline demographic and disease characteristics were balanced between the PAXLOVID and placebo groups.
The proportions of subjects who discontinued treatment due to an adverse event were 2.0% in the PAXLOVID group and 4.2% in the placebo group.
Table 9 provides results of the primary endpoint in mITT1 analysis population. For the primary endpoint, the relative risk reduction in the mITT1 analysis population for PAXLOVID compared to placebo was 86% (95% CI: 72%, 93%).
Table 9: COVID-19 Related Hospitalization or Death from Any Cause Through Day 28 in Non-Hospitalized Adults with COVID-19 (mITT1 Analysis Set): EPIC-HR PAXLOVID
(N=977)Placebo
(N=989)Abbreviations: CI=confidence interval; COVID-19=coronavirus disease 2019; mAb=monoclonal antibody; mITT1=modified intent-to-treat 1 (all treated subjects with onset of symptoms ≤5 days who at baseline did not receive nor were expected to receive COVID-19 therapeutic mAb treatment).
The determination of primary efficacy was based on a planned interim analysis of 754 subjects in mITT population. The estimated risk reduction was -6.5% with a 95% CI of (-9.3%, -3.7%) and 2-sided p-value <0.0001.- *
- The estimated cumulative proportion of subjects hospitalized or death by Day 28 was calculated for each treatment group using the Kaplan-Meier method, where subjects without hospitalization and death status through Day 28 were censored at the time of study discontinuation.
- †
- For the secondary endpoint of all-cause mortality through Week 24, there were 0 and 15 (1%) events in the PAXLOVID arm and placebo arm, respectively.
COVID-19 Related Hospitalization or Death from Any Cause Through Day 28
n (%)
9 (0.9%)
64 (6.5%)
Reduction Relative to Placebo* (95% CI), %
-5.6 (-7.3, -4.0)
COVID-19 Related Hospitalization Through Day 28, %
9 (0.9%)
63 (6.4%)
All-cause Mortality Through Day 28†, %
0
12 (1.2%)
Consistent results were observed in the mITT and mITT2 analysis populations.
Similar trends have been observed across subgroups of subjects (see Figure 1).
Figure 1: Subgroup Analysis of Adults with COVID-19 Dosed within 5 Days of Symptom Onset with COVID-19 Related Hospitalization or Death from Any Cause Through Day 28: EPIC-HR
Among subjects who were SARS-CoV-2 seropositive at baseline, 1/490 (0.2%) PAXLOVID recipients versus 8/479 (1.7%) placebo recipients met the primary endpoint of COVID-19 related hospitalization or death from any cause through Day 28 [reduction relative to placebo -1.47% (-2.70%, -0.25%)].
14.2 Trial in Unvaccinated Subjects Without a Risk Factor for Progression to Severe COVID-19 or Subjects Fully Vaccinated Against COVID-19 With at Least One Factor for Progression to Severe COVID-19 (EPIC-SR)
PAXLOVID is not indicated for the treatment of COVID-19 in patients without a risk factor for progression to severe COVID-19.
EPIC-SR (NCT05011513) was a Phase 2/3, randomized, double-blind, placebo-controlled trial in non-hospitalized symptomatic adult subjects with a laboratory confirmed diagnosis of SARS-CoV-2 infection. Eligible subjects were 18 years of age or older with COVID-19 symptom onset of ≤5 days who were at standard risk for progression to severe disease. The trial included previously unvaccinated subjects with no risk factors for progression to severe disease or subjects fully vaccinated against COVID-19 (i.e., completed a primary vaccination series) with at least 1 of the risk factors for progression to severe disease as defined in EPIC-HR. Through the December 19, 2021, data cutoff, a total of 1,075 subjects were randomized (1:1) to receive PAXLOVID or placebo orally every 12 hours for 5 days; of these, 59% were fully vaccinated high-risk subjects.
The primary endpoint in this trial, the difference in time to sustained alleviation of all targeted COVID-19 signs and symptoms through Day 28 among PAXLOVID versus placebo recipients, was not met.
In an exploratory analysis of the subgroup of fully vaccinated subjects with at least 1 risk factor for progression to severe disease, a non-statistically significant numerical reduction relative to placebo for the secondary endpoint of COVID-19 related hospitalization or death from any cause through Day 28 was observed.
Close14.3 Post-Exposure Prophylaxis Trial
PAXLOVID is not indicated for the post-exposure prophylaxis of COVID-19.
In a double-blind, double-dummy, placebo-controlled trial, the efficacy of PAXLOVID when administered for 5 or 10 days as post-exposure prophylaxis of COVID-19 was evaluated. Eligible subjects were asymptomatic adults 18 years of age and older who were SARS-CoV-2 negative at baseline and who lived in the same household with symptomatic individuals with a recent diagnosis of SARS-CoV-2. A total of 2,736 subjects were randomized (1:1:1) to receive PAXLOVID orally every 12 hours for 5 days, PAXLOVID orally every 12 hours for 10 days, or placebo.
The primary endpoint for this trial was not met. The primary endpoint was the risk reduction between the 5-day and 10-day PAXLOVID regimens versus placebo in the proportion of subjects who developed RT-PCR or RAT-confirmed symptomatic SARS-CoV-2 infection through Day 14 who had a negative SARS-CoV-2 RT-PCR result at baseline. The proportion of subjects who had events through Day 14 was 2.6% for the 5-day PAXLOVID regimen, 2.4% for the 10-day PAXLOVID regimen, and 3.9% for placebo. There was not a statistically significant risk reduction versus placebo for either the 5-day or 10-day PAXLOVID regimen.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - PAXLOVID is nirmatrelvir tablets co-packaged with ritonavir tablets. It is supplied in three different Dose Packs. Nirmatrelvir tablets and ritonavir tablets are supplied in ...
How Supplied
PAXLOVID is nirmatrelvir tablets co-packaged with ritonavir tablets. It is supplied in three different Dose Packs.
Nirmatrelvir tablets and ritonavir tablets are supplied in separate blister cavities within the same child-resistant blister card.
Dose Pack
Content
NDC
Description
300 mg nirmatrelvir; 100 mg ritonavir
Each Carton Contains:
30 tablets divided in
10 blister cards0069-5045-30
Nirmatrelvir tablets: Oval, pink immediate-release, film-coated tablets debossed with "PFE" on one side and "3CL" on the other side.
Ritonavir tablets: White to off-white, capsule-shaped, film-coated tablets debossed with "H" on one side and “R9” on the other side.
Or
0069-5321-30
Nirmatrelvir tablets: Oval, pink immediate-release, film-coated tablets debossed with "PFE" on one side and "3CL" on the other side.
Ritonavir tablets: White film-coated ovaloid tablets debossed with "NK" on one side.Each Blister Card Contains:
2 nirmatrelvir tablets
(150 mg each) and
1 ritonavir tablet
(100 mg)0069-5045-06
Nirmatrelvir tablets: Oval, pink immediate-release, film-coated tablets debossed with "PFE" on one side and "3CL" on the other side.
Ritonavir tablets: White to off-white, capsule-shaped, film-coated tablets debossed with "H" on one side and “R9” on the other side.
Or
0069-5321-03
Nirmatrelvir tablets: Oval, pink immediate-release, film-coated tablets debossed with "PFE" on one side and "3CL" on the other side.
Ritonavir tablets: White film-coated ovaloid tablets debossed with "NK" on one side.150 mg nirmatrelvir; 100 mg ritonavir
Each Carton Contains:
20 tablets divided in
10 blister cards0069-5317-20
Nirmatrelvir tablets: Oval, pink immediate-release, film-coated tablets debossed with "PFE" on one side and "3CL" on the other side.
Ritonavir tablets: White film-coated ovaloid tablets debossed with “NK” on one side.Each Blister Card Contains:
1 nirmatrelvir tablet
(150 mg) and
1 ritonavir tablet
(100 mg)0069-5317-02
Nirmatrelvir tablets: Oval, pink immediate-release, film-coated tablets debossed with "PFE" on one side and "3CL" on the other side.
Ritonavir tablets: White film-coated ovaloid tablets debossed with “NK” on one side.300 mg nirmatrelvir;
100 mg ritonavir (Day 1)150 mg nirmatrelvir;
100 mg ritonavir (Days 2-5)Each Carton Contains:
11 tablets in
1 blister card0069-0521-11
Nirmatrelvir tablets: Oval, pink immediate-release, film-coated tablets debossed with "PFE" on one side and "3CL" on the other side.
Ritonavir tablets: White film-coated ovaloid tablets debossed with “NK” on one side.Each Blister Card Contains:
6 nirmatrelvir tablets
(150 mg) and
5 ritonavir tablets
(100 mg)0069-0521-11
Nirmatrelvir tablets: Oval, pink immediate-release, film-coated tablets debossed with "PFE" on one side and "3CL" on the other side.
Ritonavir tablets: White film-coated ovaloid tablets debossed with “NK” on one side.CloseStorage and Handling
Store at USP controlled room temperature 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F).
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Drug Interactions - Inform patients that PAXLOVID may interact with certain drugs and is contraindicated for ...
Advise the patient to read the FDA-approved patient labeling (Patient Information).
CloseDrug Interactions
Inform patients that PAXLOVID may interact with certain drugs and is contraindicated for use with certain drugs; therefore, advise patients to report to their healthcare provider the use of any prescription, non-prescription medication, or herbal products [see Boxed Warning, Contraindications (4), Warnings and Precautions (5.1), and Drug Interactions (7)].
Hypersensitivity Reactions
Inform patients that anaphylaxis, serious skin reactions, and other hypersensitivity reactions have been reported, even following a single dose of PAXLOVID. Advise them to immediately discontinue the drug and to inform their healthcare provider at the first sign of a skin rash, hives or other skin reactions, difficulty in swallowing or breathing, any swelling suggesting angioedema (for example, swelling of the lips, tongue, face, tightness of the throat, hoarseness), or other symptoms of an allergic reaction [see Warnings and Precautions (5.2)].
Dosage Modification in Patients with Renal Impairment
Moderate Renal Impairment
To ensure appropriate dosing in patients with moderate renal impairment, instruct such patients that they will be taking one 150 mg nirmatrelvir tablet with one 100 mg ritonavir tablet together twice daily for 5 days [see Dosage and Administration (2.3)].
Severe Renal Impairment (Including Those Requiring Hemodialysis)
To ensure appropriate dosing in patients with severe renal impairment, including those requiring hemodialysis, instruct patients that they will be taking two 150 mg nirmatrelvir tablets with one 100 mg ritonavir tablet together once on Day 1, followed by one 150 mg nirmatrelvir tablet with one 100 mg ritonavir together once daily on Days 2-5. Instruct patients to take their dose at approximately the same time each day. On days when patients undergo hemodialysis, instruct them to take the PAXLOVID dose after hemodialysis [see Dosage and Administration (2.3)].
Administration Instructions
Inform patients to take PAXLOVID with or without food at approximately the same time each day as instructed. Advise patients to swallow all tablets for PAXLOVID whole and not to chew, break, or crush the tablets. Alert the patient of the importance of completing the full 5-day treatment course and to continuing isolation in accordance with public health recommendations to maximize viral clearance and minimize transmission of SARS-CoV-2. If the patient misses a dose of PAXLOVID within 8 hours of the time it is usually taken, the patient should take it as soon as possible and resume the normal dosing schedule. If the patient misses a dose by more than 8 hours, the patient should not take the missed dose and instead take the next dose at the regularly scheduled time. The patient should not double the dose to make up for a missed dose [see Dosage and Administration (2)].
-
SPL UNCLASSIFIED SECTIONThis product’s labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com. For Medical Information about PAXLOVID, please visit www.pfizermedinfo.com ...
This product’s labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com. For Medical Information about PAXLOVID, please visit www.pfizermedinfo.com or call 1-800-438-1985.
LAB-1523-5.0
Close -
PATIENT PACKAGE INSERTPATIENT INFORMATION - PAXLOVID (pax-LO-vid) (nirmatrelvir tablets; ritonavir tablets) co-packaged for oral use - What is the most important information I should know about ...
PATIENT INFORMATION
PAXLOVID (pax-LO-vid)
(nirmatrelvir tablets; ritonavir tablets)
co-packaged for oral useWhat is the most important information I should know about PAXLOVID?
PAXLOVID can interact with other medicines causing severe or life-threatening side effects or death. It is important to know the medicines that should not be taken with PAXLOVID.
Do not take PAXLOVID if:
- •
- you are taking any of the following medicines:
- o
- alfuzosin
- o
- amiodarone
- o
- apalutamide
- o
- carbamazepine
- o
- colchicine
- o
- dihydroergotamine
- o
- dronedarone
- o
- eletriptan
- o
- enzalutamide
- o
- eplerenone
- o
- ergotamine
- o
- finerenone
- o
- flecainide
- o
- flibanserin
- o
- ivabradine
- o
- lomitapide
- o
- lovastatin
- o
- lumacaftor/ivacaftor
- o
- lurasidone
- o
- methylergonovine
- o
- midazolam (oral)
- o
- naloxegol
- o
- phenobarbital
- o
- phenytoin
- o
- pimozide
- o
- primidone
- o
- propafenone
- o
- quinidine
- o
- ranolazine
- o
- rifampin
- o
- rifapentine
- o
- St. John’s Wort (hypericum perforatum)
- o
- sildenafil (Revatio®) for pulmonary arterial hypertension
- o
- silodosin
- o
- simvastatin
- o
- tolvaptan
- o
- triazolam
- o
- ubrogepant
- o
- voclosporin
These are not the only medicines that may cause serious or life-threatening side effects if taken with PAXLOVID. PAXLOVID may increase or decrease the levels of multiple other medicines. It is very important to tell your healthcare provider about all of the medicines you are taking because additional laboratory tests or changes in the dose of your other medicines may be necessary during treatment with PAXLOVID. Your healthcare provider may also tell you about specific symptoms to watch out for that may indicate that you need to stop or decrease the dose of some of your other medicines.
- •
- you are allergic to nirmatrelvir, ritonavir, or any of the ingredients in PAXLOVID. See the end of this leaflet for a complete list of ingredients in PAXLOVID. See “What are the possible side effects of PAXLOVID?” for signs and symptoms of allergic reactions.
What is PAXLOVID?
PAXLOVID is a prescription medicine used to treat mild-to-moderate coronavirus disease 2019 (COVID-19) in adults who are at high risk for progression to severe COVID-19, including hospitalization or death.PAXLOVID is not approved for use as pre-exposure or post-exposure treatment for prevention of COVID-19.
Before taking PAXLOVID, tell your healthcare provider about all of your medical conditions, including if you:
- •
- have kidney problems. You may need a different dose or dosing schedule of PAXLOVID.
- •
- have liver problems, including hepatitis.
- •
- have Human Immunodeficiency Virus 1 (HIV-1) infection. PAXLOVID may lead to some HIV-1 medicines not working as well in the future.
- •
- are pregnant or plan to become pregnant. It is not known if PAXLOVID can harm your unborn baby. Tell your healthcare provider right away if you are or if you become pregnant.
- •
- are breastfeeding or plan to breastfeed. PAXLOVID can pass into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with PAXLOVID.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
- •
- Your healthcare provider can tell you if it is safe to take PAXLOVID with other medicines.
- •
- You can ask your healthcare provider or pharmacist for a list of medicines that interact with PAXLOVID.
- •
- Do not start taking a new medicine without telling your healthcare provider.
Tell your healthcare provider if you are taking combined birth control (hormonal contraceptive). PAXLOVID may affect how your hormonal contraceptives work. Females who are able to become pregnant should use another effective alternative form of contraception or an additional barrier method of contraception during treatment with PAXLOVID. Talk to your healthcare provider if you have any questions about contraceptive methods that might be right for you.
How should I take PAXLOVID?
- •
- Take PAXLOVID exactly as your healthcare provider tells you to take it.
- •
-
PAXLOVID consists of 2 medicines: nirmatrelvir tablets and ritonavir tablets. The 2 medicines are taken together for 5 days.
- o
- Nirmatrelvir is an oval, pink tablet.
- o
- Ritonavir is a white or off-white tablet.
- •
- PAXLOVID is available in 3 Dose Packs (see Figures A, B, and C below). Your healthcare provider will prescribe the PAXLOVID Dose Pack that is right for you. Follow the instruction for the Dose Pack you receive.
- •
- If you have kidney disease, your healthcare provider may prescribe a lower dose (see Figures B and C). Talk to your healthcare provider to make sure you receive the correct Dose Pack.
If you are prescribed PAXLOVID 300 mg; 100 mg Dose Pack
Each dose contains 3 tablets taken together twice daily
How to take PAXLOVID 300 mg; 100 mg Dose Pack
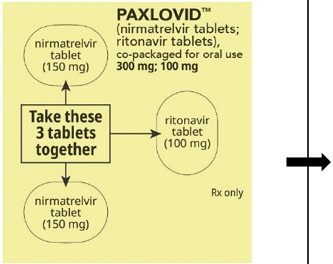
Morning Dose:
Take the 2 pink nirmatrelvir tablets and
1 white to off-white ritonavir tablet together.

Bedtime Dose:
Take the 2 pink nirmatrelvir tablets and
1 white to off-white ritonavir tablet together.
If you are prescribed PAXLOVID 150 mg; 100 mg Dose Pack
Each dose contains 2 tablets taken together twice daily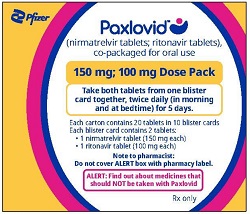
How to take PAXLOVID 150 mg; 100 mg Dose Pack
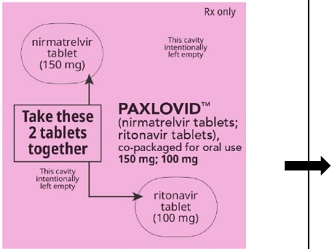
Morning Dose:
Take the 1 pink nirmatrelvir tablet and
1 white ritonavir tablet together.

Bedtime Dose:
Take the 1 pink nirmatrelvir tablet and
1 white ritonavir tablet together.
If you are prescribed PAXLOVID 300 mg; 100 mg (Day 1) and 150 mg; 100 mg (Days 2-5)
Each dose is taken together once daily; on days of dialysis take PAXLOVID after receiving dialysis
How to take PAXLOVID 300 mg; 100 mg (Day 1) and 150 mg; 100 mg (Days 2-5)

Day 1 (First Day):
Take the 2 pink nirmatrelvir tablets and
1 white ritonavir tablet together
(Blue part of the blister card).

Days 2-5:
Take the 1 pink nirmatrelvir tablet and
1 white ritonavir tablet together
- •
- Do not remove your PAXLOVID tablets from the blister card before you are ready to take your dose.
- •
- If you are taking PAXLOVID tablets twice daily (Figure A or Figure B), take your first dose of PAXLOVID in the morning or at bedtime, depending on when you pick up your prescription, or as your healthcare provider tells you to. Take your doses at around the same time each day.
- •
- If you have severe kidney disease and are taking PAXLOVID tablets once daily (Figure C), follow the daily dose instruction on the blister card. Take your dose at around the same time each day.
- •
- Swallow the tablets whole. Do not chew, break, or crush the tablets.
- •
- Take PAXLOVID with or without food.
- •
- Do not stop taking PAXLOVID without talking to your healthcare provider, even if you feel better.
- •
- If you miss a dose of PAXLOVID within 8 hours of the time it is usually taken, take it as soon as you remember. If you miss a dose by more than 8 hours, skip the missed dose and take the next dose at your regular time. Do not take 2 doses of PAXLOVID at the same time.
- •
- If you take too much PAXLOVID, call your healthcare provider or go to the nearest hospital emergency room right away.
- •
- If you are taking a ritonavir- or cobicistat-containing medicine to treat hepatitis C or HIV-1 infection, you should continue to take your medicine as prescribed by your healthcare provider.
Talk to your healthcare provider if you do not feel better or if you feel worse after 5 days.
What are the possible side effects of PAXLOVID?
PAXLOVID may cause serious side effects, including:
- •
- Allergic reactions, including severe allergic reactions (anaphylaxis) have happened during treatment with PAXLOVID. Stop taking PAXLOVID and get medical help right away if you get any of the following symptoms of an allergic reaction:
- o
- skin rash, hives, blisters or peeling skin
- o
- painful sores or ulcers in the mouth, nose, throat or genital area
- o
- swelling of the mouth, lips, tongue or face
- o
- trouble swallowing or breathing
- o
- throat tightness
- o
- hoarseness
- •
- Liver problems. Tell your healthcare provider right away if you get any of the following signs and symptoms of liver problems during treatment with PAXLOVID:
- o
- loss of appetite
- o
- yellowing of your skin and the white of eyes
- o
- dark-colored urine
- o
- pale colored stools
- o
- itchy skin
- o
- stomach-area (abdominal) pain
The most common side effects of PAXLOVID include: altered sense of taste (such as metallic, bitter taste) and diarrhea.
Other possible side effects include:
- •
- headache
- •
- vomiting
- •
- abdominal pain
- •
- nausea
- •
- high blood pressure
- •
- feeling generally unwell
These are not all of the possible side effects of PAXLOVID. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store PAXLOVID?
- •
- Store PAXLOVID at room temperature between 68°F to 77°F (20°C to 25°C).
Keep PAXLOVID and all medicines out of the reach of children.
General information about the safe and effective use of PAXLOVID.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use PAXLOVID for a condition for which it was not prescribed. Do not give PAXLOVID to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for more information about PAXLOVID that is written for health professionals.What are the ingredients in PAXLOVID?
Active ingredient: nirmatrelvir and ritonavir
Nirmatrelvir inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, lactose monohydrate, microcrystalline cellulose, and sodium stearyl fumarate. Film-coating contains: hydroxy propyl methylcellulose, iron oxide red, polyethylene glycol, and titanium dioxide.
Ritonavir inactive ingredients: anhydrous dibasic calcium phosphate, colloidal silicon dioxide, copovidone, sodium stearyl fumarate, and sorbitan monolaurate. The film coating may contain: colloidal anhydrous silica, colloidal silicon dioxide, hydroxypropyl cellulose, hypromellose, polyethylene glycol, polysorbate 80, talc, and titanium dioxide.
LAB-1524-4.0
For more information, go to www.pfizer.com or call 1-800-438-1985.This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 02/2025
Close -
PRINCIPAL DISPLAY PANEL - 3 Tablet Blister Pack - 0069-5045NDC 0069-5045-06 - PAXLOVID™ (nirmatrelvir tablets; ritonavir tablets), co-packaged for oral use - 300 mg; 100 mg - nirmatrelvir - tablet - (150 mg) Take these - 3 tablets - together - ritonavir - tablet - (100 ...
NDC 0069-5045-06
PAXLOVID™
(nirmatrelvir tablets;
ritonavir tablets),
co-packaged for oral use
300 mg; 100 mgnirmatrelvir
tablet
(150 mg)Take these
3 tablets
togetherritonavir
tablet
(100 mg)nirmatrelvir
tablet
(150 mg)Rx only
PAA203000
Dist. by Pfizer Labs
Div. of Pfizer Inc., NY, NY 10001(01)10300695045063
LOT:
Close
XXXXXXX
EXP:
MM/YYYY -
PRINCIPAL DISPLAY PANEL - Kit Carton - 0069-5045 Pfizer - NDC 0069-5045-30 - PAXLOVID™ (nirmatrelvir tablets; ritonavir tablets), co-packaged for oral use - 300 mg; 100 mg Dose Pack - Take all 3 tablets from one blister - card together, twice daily (in ...
Pfizer
NDC 0069-5045-30PAXLOVID™
(nirmatrelvir tablets; ritonavir tablets),
co-packaged for oral use300 mg; 100 mg Dose Pack
Take all 3 tablets from one blister
card together, twice daily (in morning
and at bedtime) for 5 days.Each carton contains 30 tablets in 10 blister cards
Each blister card contains 3 tablets:- •
- 2 nirmatrelvir tablets (150 mg each)
- •
- 1 ritonavir tablet (100 mg each)
Note to pharmacist:
Do not cover ALERT box with pharmacy label.ALERT: Find out about medicines that
should NOT be taken with PaxlovidRx only
Close -
PRINCIPAL DISPLAY PANEL - 3 Tablet Blister Pack - 0069-5321 NDC 0069-5321-03 - PAXLOVID™ (nirmatrelvir tablets; ritonavir tablets), co-packaged for oral use - 300 mg; 100 mg - nirmatrelvir - tablet - (150 mg) Take these - 3 tablets - together - ritonavir - tablet - (100 ...
NDC 0069-5321-03
PAXLOVID™
(nirmatrelvir tablets;
ritonavir tablets),
co-packaged for oral use
300 mg; 100 mgnirmatrelvir
tablet
(150 mg)Take these
3 tablets
togetherritonavir
tablet
(100 mg)nirmatrelvir
tablet
(150 mg)Rx only
PAA206981
Dist. by Pfizer Labs
Div. of Pfizer Inc., NY, NY 10001(01)10300695321037
LOT:
Close
EXP: -
PRINCIPAL DISPLAY PANEL - Kit Carton - 0069-5321 Pfizer - NDC 0069-5321-30 - PAXLOVID™ (nirmatrelvir tablets; ritonavir tablets), co-packaged for oral use - 300 mg; 100 mg Dose Pack - Take all 3 tablets from one blister - card together, twice daily (in ...
Pfizer
NDC 0069-5321-30PAXLOVID™
(nirmatrelvir tablets; ritonavir tablets),
co-packaged for oral use300 mg; 100 mg Dose Pack
Take all 3 tablets from one blister
card together, twice daily (in morning
and at bedtime) for 5 days.Each carton contains 30 tablets in 10 blister cards
Each blister card contains 3 tablets:- •
- 2 nirmatrelvir tablets (150 mg each)
- •
- 1 ritonavir tablet (100 mg each)
Note to pharmacist:
Do not cover ALERT box with pharmacy label.ALERT: Find out about medicines that
should NOT be taken with PaxlovidRx only
Close -
PRINCIPAL DISPLAY PANEL - 2 Tablet Blister Pack - 0069-5317 NDC 0069-5317-02 - Rx only - nirmatrelvir - tablet - (150 mg) This cavity - intentionally - left empty - Take these - 2 tablets - together - PAXLOVID™ (nirmatrelvir tablets; ritonavir tablets), co-packaged for oral ...
NDC 0069-5317-02
Rx onlynirmatrelvir
tablet
(150 mg)This cavity
intentionally
left emptyTake these
2 tablets
togetherPAXLOVID™
(nirmatrelvir tablets;
ritonavir tablets),
co-packaged for oral use
150 mg; 100 mgThis cavity
intentionally
left emptyritonavir
tablet
(100 mg)Dist. by Pfizer Labs
Div. of Pfizer Inc., NY, NY 10001(01)10300695317023
LOT:
Close
EXP:
PAA206982 -
PRINCIPAL DISPLAY PANEL - Kit Carton - 0069-5317 Pfizer - NDC 0069-5317-20 - PAXLOVID™ (nirmatrelvir tablets; ritonavir tablets), co-packaged for oral use - 150 mg; 100 mg Dose Pack - Take both tablets from one blister - card together, twice daily (in ...
Pfizer
NDC 0069-5317-20PAXLOVID™
(nirmatrelvir tablets; ritonavir tablets),
co-packaged for oral use150 mg; 100 mg Dose Pack
Take both tablets from one blister
card together, twice daily (in morning
and at bedtime) for 5 days.Each carton contains 20 tablets in 10 blister cards
Each blister card contains 2 tablets:- •
- 1 nirmatrelvir tablet (150 mg each)
- •
- 1 ritonavir tablet (100 mg each)
Note to pharmacist:
Do not cover ALERT box with pharmacy label.ALERT: Find out about medicines that
should NOT be taken with PaxlovidRx only
Close -
PRINCIPAL DISPLAY PANEL - 11 Tablet Blister Pack - 0069-0521 Day 1 (300 mg; 100 mg) Your first day’s dose. Take these 3 tablets together - nirmatrelvir - tablet - (150 mg) nirmatrelvir - tablet - (150 mg) ritonavir - tablet - (100 mg) NDC 0069-0521-11 - Rx ...
Day 1 (300 mg; 100 mg)
Your first day’s dose. Take these 3 tablets togethernirmatrelvir
tablet
(150 mg)
nirmatrelvir
tablet
(150 mg)
ritonavir
tablet
(100 mg)NDC 0069-0521-11
Rx only
PAXLOVID™
(nirmatrelvir tablets; ritonavir tablets), co-packaged for oral usenirmatrelvir
tablet
(150 mg)
Day 2 (150 mg; 100 mg)
Take these
2 tablets together
ritonavir
tablet
(100 mg)PAA228321
nirmatrelvir
tablet
(150 mg)
Day 3 (150 mg; 100 mg)
Take these
2 tablets together
ritonavir
tablet
(100 mg)nirmatrelvir
tablet
(150 mg)
Day 4 (150 mg; 100 mg)
Take these
2 tablets together
ritonavir
tablet
(100 mg)nirmatrelvir
tablet
(150 mg)
Day 5 (150 mg; 100 mg)
Take these
2 tablets together
ritonavir
tablet
(100 mg)Dist. by Pfizer Labs, Div. of Pfizer Inc., NY, NY 10001
Close -
PRINCIPAL DISPLAY PANEL – Kit Carton - 0069-0521 Pfizer - NDC 0069-0521-11 - PAXLOVID™ (nirmatrelvir tablets; ritonavir tablets), co-packaged for oral use - 300 mg; 100 mg (Day 1) 150 mg; 100 mg (Days 2 – 5) Day 1: Take 2 nirmatrelvir tablets and - 1 ...
Pfizer
NDC 0069-0521-11PAXLOVID™
(nirmatrelvir tablets; ritonavir tablets),
co-packaged for oral use300 mg; 100 mg (Day 1)
150 mg; 100 mg (Days 2 – 5)Day 1:
Take 2 nirmatrelvir tablets and
1 ritonavir tablet together
Days 2 - 5:
Take 1 nirmatrelvir tablet and
1 ritonavir tablet togetherCarton contains 1 blister card with 11 tablets:
- •
- 6 nirmatrelvir tablets (150 mg each)
- •
- 5 ritonavir tablets (100 mg each)
Note to pharmacist:
Do not cover ALERT box with pharmacy label.ALERT: Find out about medicines that
should NOT be taken with PaxlovidRx only
Close -
INGREDIENTS AND APPEARANCEProduct Information
PAXLOVID nirmatrelvir and ritonavir kit Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0069-5045 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-5045-30 10 in 1 CARTON 05/25/2023 05/25/2023 1 NDC:0069-5045-06 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 10 BLISTER PACK 20 Part 2 10 BLISTER PACK 10 Part 1 of 2 NIRMATRELVIR nirmatrelvir tablet, film coated Product Information Item Code (Source) NDC:0069-2085 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NIRMATRELVIR (UNII: 7R9A5P7H32) (NIRMATRELVIR - UNII:7R9A5P7H32) NIRMATRELVIR 150 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) HYPROMELLOSE 2910 (10000 MPA.S) (UNII: 0HO1H52958) FERRIC OXIDE RED (UNII: 1K09F3G675) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color PINK Score no score Shape OVAL Size 18mm Flavor Imprint Code PFE;3CL Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-2085-02 2 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217188 05/25/2023 05/25/2023 Part 2 of 2 RITONAVIR ritonavir tablet, film coated Product Information Item Code (Source) NDC:0069-1345 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RITONAVIR (UNII: O3J8G9O825) (RITONAVIR - UNII:O3J8G9O825) RITONAVIR 100 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) COPOVIDONE K25-31 (UNII: D9C330MD8B) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) SORBITAN MONOLAURATE (UNII: 6W9PS8B71J) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (white to off-white) Score no score Shape OVAL (Capsule) Size 18mm Flavor Imprint Code H;R9 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-1345-11 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217188 05/25/2023 05/25/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217188 05/25/2023 05/25/2023 PAXLOVID nirmatrelvir and ritonavir kit Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0069-5321 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-5321-30 10 in 1 CARTON 10/18/2023 1 NDC:0069-5321-03 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 10 BLISTER PACK 20 Part 2 10 BLISTER PACK 10 Part 1 of 2 NIRMATRELVIR nirmatrelvir tablet, film coated Product Information Item Code (Source) NDC:0069-2085 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NIRMATRELVIR (UNII: 7R9A5P7H32) (NIRMATRELVIR - UNII:7R9A5P7H32) NIRMATRELVIR 150 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) HYPROMELLOSE 2910 (10000 MPA.S) (UNII: 0HO1H52958) FERRIC OXIDE RED (UNII: 1K09F3G675) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color PINK Score no score Shape OVAL Size 18mm Flavor Imprint Code PFE;3CL Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-2085-02 2 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217188 10/18/2023 Part 2 of 2 RITONAVIR ritonavir tablet, film coated Product Information Item Code (Source) NDC:0069-1735 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RITONAVIR (UNII: O3J8G9O825) (RITONAVIR - UNII:O3J8G9O825) RITONAVIR 100 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) COPOVIDONE K25-31 (UNII: D9C330MD8B) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) SORBITAN MONOLAURATE (UNII: 6W9PS8B71J) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE Score no score Shape OVAL (ovaloid) Size 17mm Flavor Imprint Code NK Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-1735-11 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217188 10/18/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217188 10/18/2023 PAXLOVID nirmatrelvir and ritonavir kit Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0069-5317 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-5317-20 10 in 1 CARTON 10/18/2023 1 NDC:0069-5317-02 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 10 BLISTER PACK 10 Part 2 10 BLISTER PACK 10 Part 1 of 2 NIRMATRELVIR nirmatrelvir tablet, film coated Product Information Item Code (Source) NDC:0069-2085 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NIRMATRELVIR (UNII: 7R9A5P7H32) (NIRMATRELVIR - UNII:7R9A5P7H32) NIRMATRELVIR 150 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) HYPROMELLOSE 2910 (10000 MPA.S) (UNII: 0HO1H52958) FERRIC OXIDE RED (UNII: 1K09F3G675) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color PINK Score no score Shape OVAL Size 18mm Flavor Imprint Code PFE;3CL Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-2085-11 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217188 10/18/2023 Part 2 of 2 RITONAVIR ritonavir tablet, film coated Product Information Item Code (Source) NDC:0069-1735 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RITONAVIR (UNII: O3J8G9O825) (RITONAVIR - UNII:O3J8G9O825) RITONAVIR 100 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) COPOVIDONE K25-31 (UNII: D9C330MD8B) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) SORBITAN MONOLAURATE (UNII: 6W9PS8B71J) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE Score no score Shape OVAL (ovaloid) Size 17mm Flavor Imprint Code NK Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-1735-11 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217188 10/18/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217188 10/18/2023 PAXLOVID nirmatrelvir and ritonavir kit Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0069-0521 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-0521-11 1 in 1 CARTON 04/15/2025 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BLISTER PACK 6 Part 2 1 BLISTER PACK 5 Part 1 of 2 NIRMATRELVIR nirmatrelvir tablet, film coated Product Information Item Code (Source) NDC:0069-2085 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NIRMATRELVIR (UNII: 7R9A5P7H32) (NIRMATRELVIR - UNII:7R9A5P7H32) NIRMATRELVIR 150 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) HYPROMELLOSE 2910 (10000 MPA.S) (UNII: 0HO1H52958) FERRIC OXIDE RED (UNII: 1K09F3G675) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color PINK Score no score Shape OVAL Size 18mm Flavor Imprint Code PFE;3CL Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-2085-06 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217188 04/15/2025 Part 2 of 2 RITONAVIR ritonavir tablet, film coated Product Information Item Code (Source) NDC:0069-1735 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RITONAVIR (UNII: O3J8G9O825) (RITONAVIR - UNII:O3J8G9O825) RITONAVIR 100 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) COPOVIDONE K25-31 (UNII: D9C330MD8B) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) SORBITAN MONOLAURATE (UNII: 6W9PS8B71J) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE Score no score Shape OVAL (ovaloid) Size 17mm Flavor Imprint Code NK Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-1735-05 5 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217188 04/15/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217188 04/15/2025 Labeler - Pfizer Laboratories Div Pfizer Inc (134489525) Registrant - Pfizer Inc (113480771) Establishment Name Address ID/FEI Business Operations Pfizer Ireland Pharmaceuticals Unlimited Company 985052076 ANALYSIS(0069-5045, 0069-5321, 0069-5317, 0069-0521) , API MANUFACTURE(0069-5045, 0069-5321, 0069-5317, 0069-0521) Establishment Name Address ID/FEI Business Operations Pfizer Manufacturing Deutschland GmbH 341970073 ANALYSIS(0069-5045, 0069-5321, 0069-5317) , MANUFACTURE(0069-5045, 0069-5321, 0069-5317) , PACK(0069-5045, 0069-5321, 0069-5317) , LABEL(0069-5045, 0069-5321, 0069-5317) Establishment Name Address ID/FEI Business Operations Pfizer Ireland Pharmaceuticals Unlimited Company 986019327 ANALYSIS(0069-5045, 0069-5321, 0069-5317, 0069-0521) , MANUFACTURE(0069-5045, 0069-5321, 0069-5317, 0069-0521) , PACK(0069-5045, 0069-5321, 0069-5317, 0069-0521) , LABEL(0069-5045, 0069-5321, 0069-5317, 0069-0521) Establishment Name Address ID/FEI Business Operations Pfizer Italia S.r.l. 458521908 ANALYSIS(0069-5045, 0069-5321, 0069-5317) , MANUFACTURE(0069-5045, 0069-5321, 0069-5317) , PACK(0069-5045, 0069-5321, 0069-5317) , LABEL(0069-5045, 0069-5321, 0069-5317)
CloseEstablishment Name Address ID/FEI Business Operations Pfizer Inc 943955690 ANALYSIS(0069-5045, 0069-5321, 0069-5317)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
PAXLOVID- nirmatrelvir and ritonavir kit
Number of versions: 8
RxNorm
PAXLOVID- nirmatrelvir and ritonavir kit
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 900575 | ritonavir 100 MG Oral Tablet | PSN |
| 2 | 900575 | ritonavir 100 MG Oral Tablet | SCD |
| 3 | 2587897 | nirmatrelvir 150 MG Oral Tablet | PSN |
| 4 | 2587897 | nirmatrelvir 150 MG Oral Tablet | SCD |
| 5 | 2587898 | nirmatrelvir 300 MG ; ritonavir 100 MG Daily Dose Pack | PSN |
| 6 | 2587898 | {20 (nirmatrelvir 150 MG Oral Tablet) / 10 (ritonavir 100 MG Oral Tablet) } Pack | GPCK |
| 7 | 2587898 | nirmatrelvir 150 MG Oral Tablet (20) / ritonavir 100 MG Oral Tablet (10) 5-Day Pack | SY |
| 8 | 2587899 | PAXLOVID 300 MG ; 100 MG Dose Pack | PSN |
| 9 | 2587899 | {20 (nirmatrelvir 150 MG Oral Tablet) / 10 (ritonavir 100 MG Oral Tablet) } Pack [Paxlovid 5-Day] | BPCK |
| 10 | 2587899 | Paxlovid 300 MG / 100 MG Dose Pack | SY |
| 11 | 2599542 | nirmatrelvir 150 MG ; ritonavir 100 MG Daily Dose Pack, Renal | PSN |
| 12 | 2599542 | {10 (nirmatrelvir 150 MG Oral Tablet) / 10 (ritonavir 100 MG Oral Tablet) } Pack | GPCK |
| 13 | 2599542 | nirmatrelvir 150 MG Oral Tablet (10) / ritonavir 100 MG Oral Tablet (10) 150 MG /100 MG Dose Pack | SY |
| 14 | 2599543 | PAXLOVID 150 MG ; 100 MG Dose Pack, Renal | PSN |
| 15 | 2599543 | {10 (nirmatrelvir 150 MG Oral Tablet) / 10 (ritonavir 100 MG Oral Tablet) } Pack [Paxlovid 150 MG /100 MG Dose Pack] | BPCK |
| 16 | 2599543 | Paxlovid 150 MG / 100 MG Dose Pack | SY |
| 17 | 2704986 | nirmatrelvir / ritonavir 300 MG /100 MG ; 150 MG / 100 MG Dose Pack | PSN |
| 18 | 2704986 | {6 (nirmatrelvir 150 MG Oral Tablet) / 5 (ritonavir 100 MG Oral Tablet) } Pack | GPCK |
| 19 | 2704986 | nirmatrelvir 150 MG Oral Tablet (6) / ritonavir 100 MG Oral Tablet (5) 300 MG /100 MG ; 150 MG / 100 MG Dose Pack | SY |
| 20 | 2704987 | PAXLOVID 300 MG ; 100 MG (Day 1), 150 MG ; 100 MG (Days 2-5) Dose Pack | PSN |
| 21 | 2704987 | {6 (nirmatrelvir 150 MG Oral Tablet) / 5 (ritonavir 100 MG Oral Tablet) } Pack [Paxlovid 300 MG /100 MG ; 150 MG / 100 MG Dose Pack] | BPCK |
| 22 | 2704987 | Paxlovid 300 MG / 100 MG ; 150 MG / 100 MG Dose Pack | SY |
Get Label RSS Feed for this Drug
PAXLOVID- nirmatrelvir and ritonavir kit
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=8a99d6d6-fd9e-45bb-b1bf-48c7f761232a
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
PAXLOVID- nirmatrelvir and ritonavir kit
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 0069-0521-11 |
| 2 | 0069-1345-11 |
| 3 | 0069-1735-05 |
| 4 | 0069-1735-11 |
| 5 | 0069-2085-02 |
| 6 | 0069-2085-06 |
| 7 | 0069-2085-11 |
| 8 | 0069-5045-06 |
| 9 | 0069-5045-30 |
| 10 | 0069-5317-02 |
| 11 | 0069-5317-20 |
| 12 | 0069-5321-03 |
| 13 | 0069-5321-30 |