Label: SUBOXONE- buprenorphine hydrochloride, naloxone hydrochloride film, soluble
-
NDC Code(s):
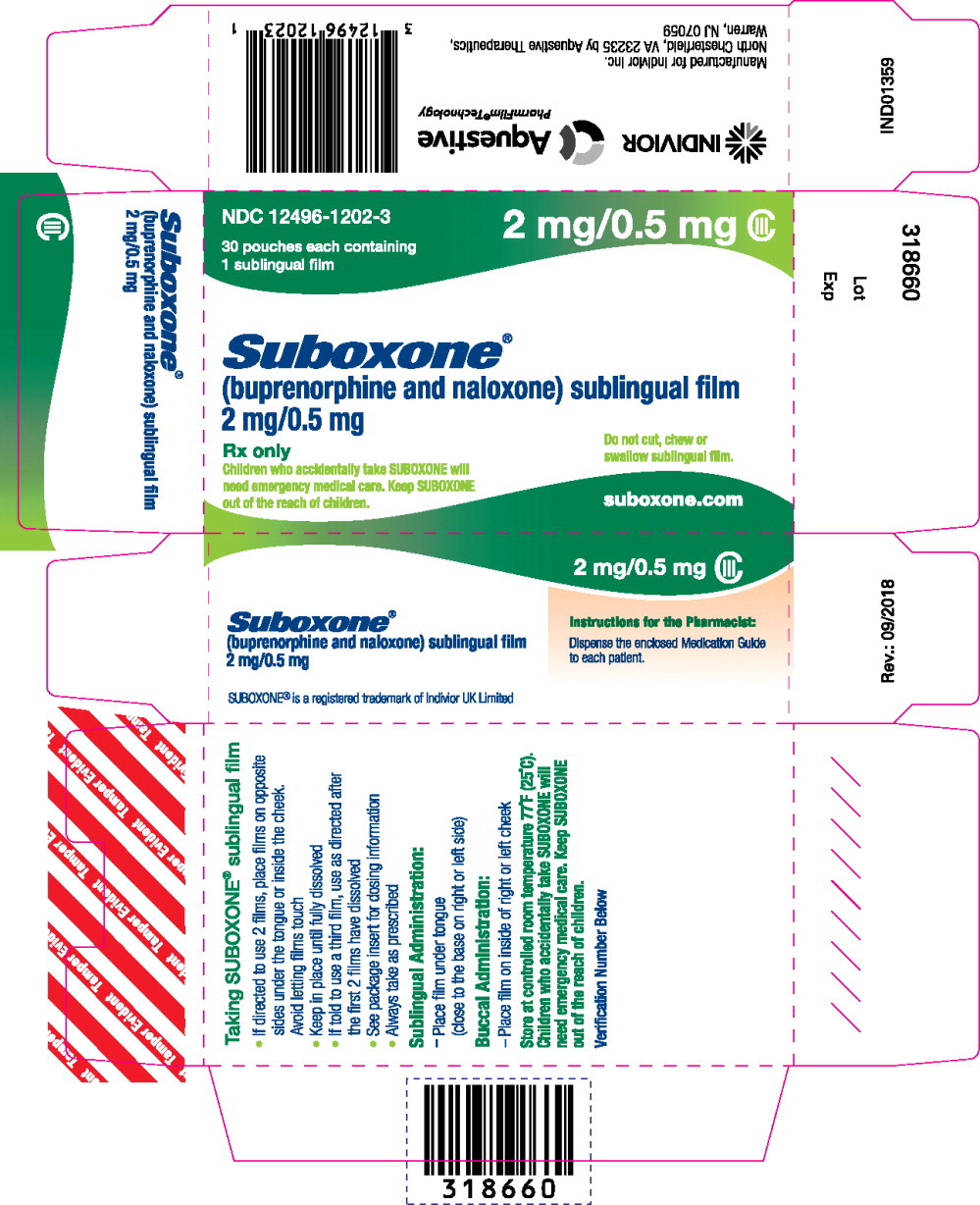
12496-1202-1,
12496-1202-3,
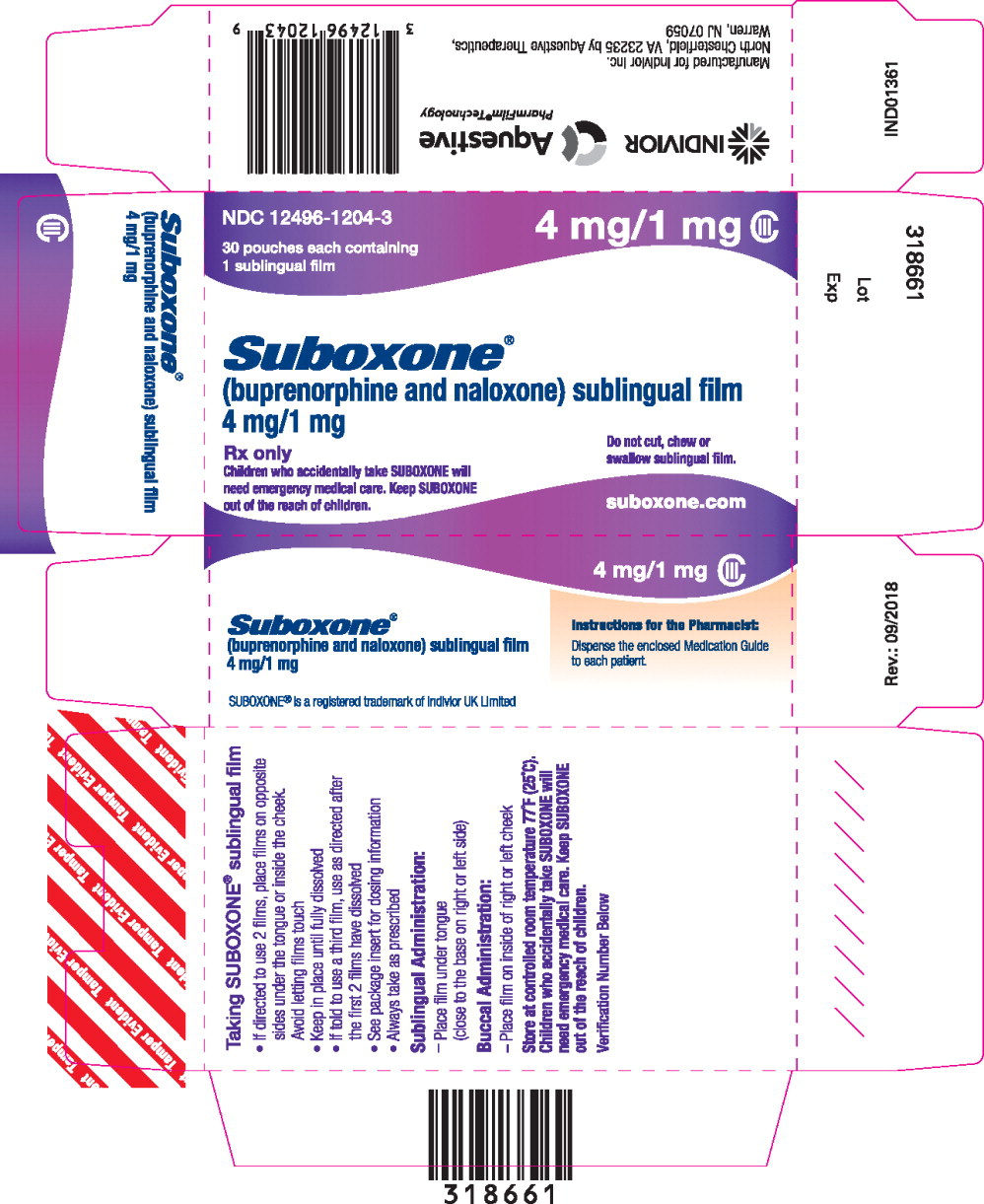
12496-1204-1,
12496-1204-3, view more12496-1208-1, 12496-1208-3, 12496-1212-1, 12496-1212-3
- Packager: INDIVIOR INC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIII
- Marketing Status: New Drug Application
Drug Label Information
Updated November 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SUBOXONE sublingual film safely and effectively. See full prescribing information for SUBOXONE sublingual film. SUBOXONE (buprenorphine and naloxone) sublingual film, for sublingual or buccal use, CIII Initial U.S. Approval: 2002
INDICATIONS AND USAGE
SUBOXONE® sublingual film contains buprenorphine, a partial‐opioid agonist, and naloxone, an opioid antagonist, and is indicated for treatment of opioid dependence. ( 1)
SUBOXONE sublingual film should be used as part of a complete treatment plan that includes counseling and psychosocial support. ( 1)
DOSAGE AND ADMINISTRATION
- Administer SUBOXONE sublingual film as a single daily dose. ( 2.1)
- Strongly consider prescribing naloxone at the time SUBOXONE sublingual film is initiated or renewed because patients being treated for opioid use disorder have the potential for relapse, putting them at risk for opioid overdose. ( 2.2)
- To avoid precipitating withdrawal, induction with SUBOXONE sublingual film should be undertaken when objective and clear signs of withdrawal are evident and SUBOXONE sublingual film should be administered in divided doses when used as initial treatment. ( 2.3)
- For patients dependent on short‐acting opioid products who are in opioid withdrawal; on Day 1, administer up to 8 mg/2 mg SUBOXONE sublingual film (in divided doses). On Day 2, administer up to 16 mg/4 mg of SUBOXONE sublingual film as a single dose. ( 2.3)
- For patients dependent on methadone or long‐acting opioid products, induction onto sublingual buprenorphine monotherapy is recommended on Days 1 and 2 of treatment. ( 2.3)
- For maintenance treatment, the target dosage of SUBOXONE sublingual film is usually 16 mg/4 mg as a single daily dose. ( 2.4)
- Sublingual Administration: Place one film under the tongue, close to the base on the left or right side, and allow to completely dissolve. Buccal Administration: Place one film on the inside of the left or right cheek and allow to completely dissolve. ( 2.5)
- SUBOXONE sublingual film must be administered whole. Do not cut, chew, or swallow SUBOXONE sublingual film ( 2.5)
- When discontinuing treatment, gradually taper to avoid signs and symptoms of withdrawal. ( 2.8)
DOSAGE FORMS AND STRENGTHS
Sublingual film:
- buprenorphine 2 mg/ naloxone 0.5 mg,
- buprenorphine 4 mg/ naloxone 1 mg,
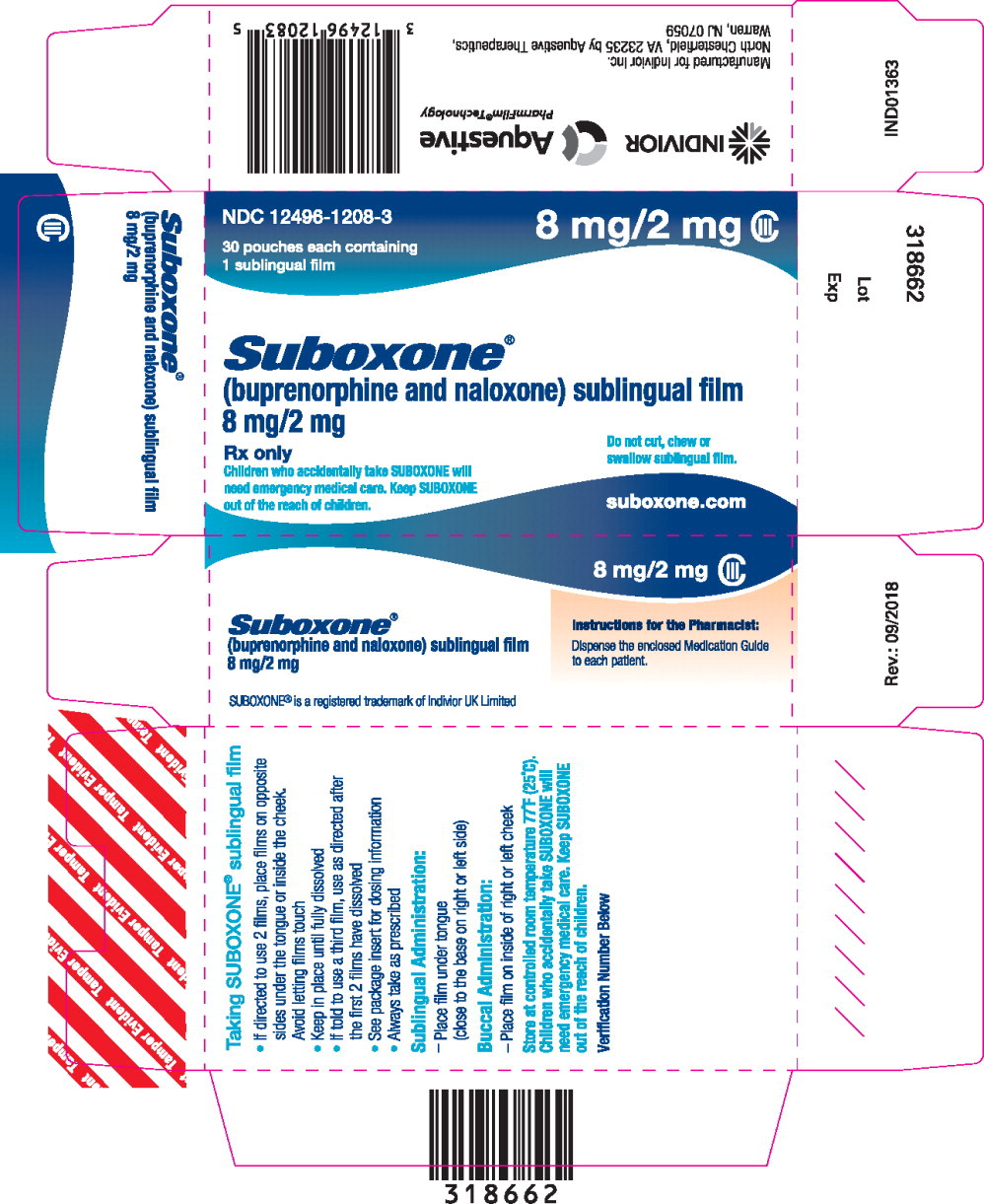
- buprenorphine 8 mg/ naloxone 2 mg and
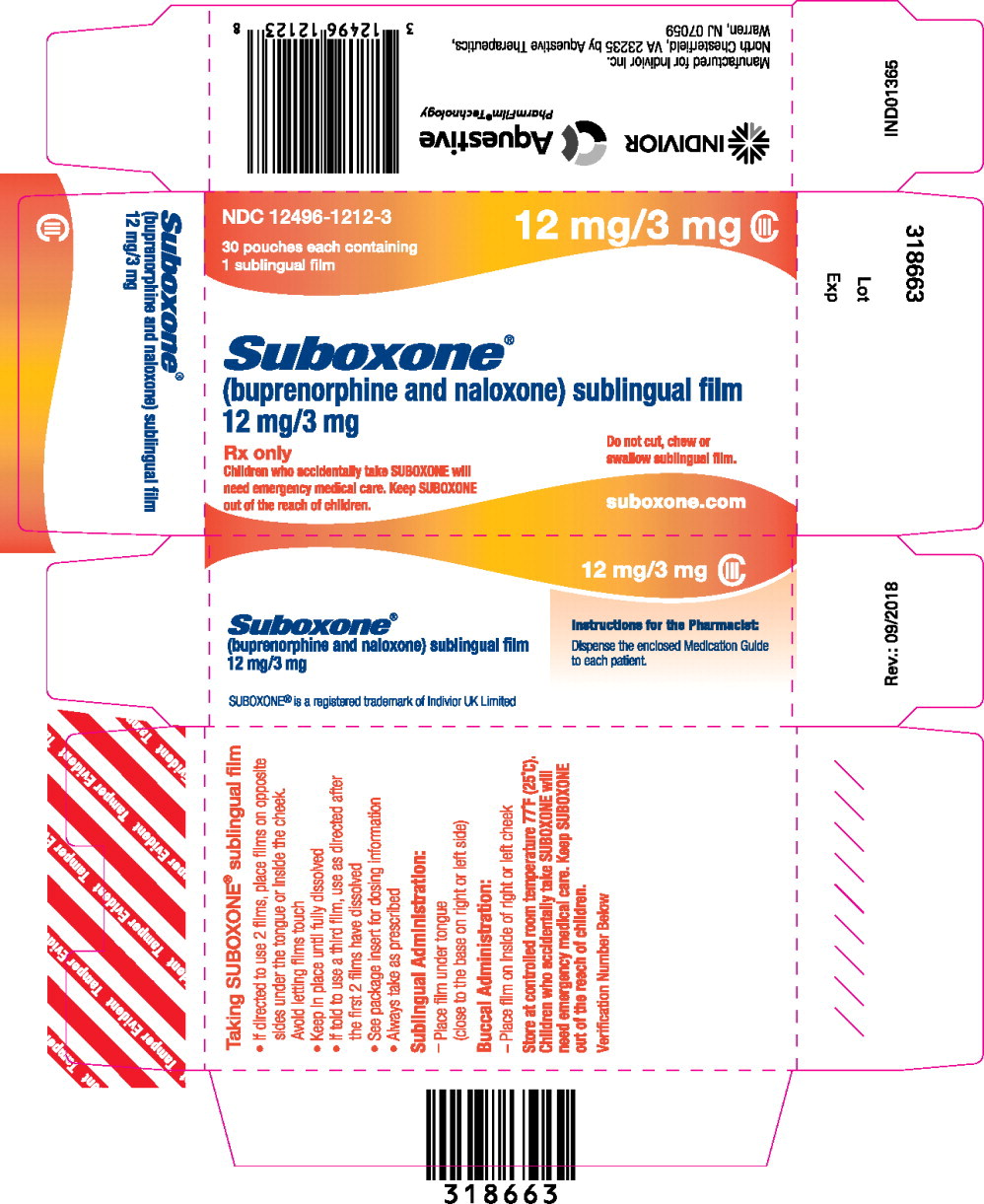
- buprenorphine 12 mg/ naloxone 3 mg. ( 3)
CONTRAINDICATIONS
Hypersensitivity to buprenorphine or naloxone. ( 4)
WARNINGS AND PRECAUTIONS
- Addiction, Abuse, and Misuse:Buprenorphine can be abused in a similar manner to other opioids. Monitor patients for conditions indicative of diversion or progression of opioid dependence and addictive behaviors. Multiple refills should not be prescribed early in treatment or without appropriate patient follow-up visits. ( 5.1)
- Respiratory Depression:Life-threatening respiratory depression and death have occurred in association with buprenorphine use. Warn patients of the potential danger of self-administration of benzodiazepines or other CNS depressants while under treatment with SUBOXONE sublingual film. ( 5.2, 5.3)
- Unintentional Pediatric Exposure:Store SUBOXONE sublingual film safely out of the sight and reach of children. Buprenorphine can cause severe, possibly fatal, respiratory depression in children. ( 5.4)
- Neonatal Opioid Withdrawal Syndrome:Neonatal opioid withdrawal syndrome (NOWS) is an expected and treatable outcome of prolonged use of opioids during pregnancy ( 5.5)
- Adrenal Insufficiency:If diagnosed, treat with physiologic replacement of corticosteroids, and wean patient off of the opioid. ( 5.6)
- Risk of Opioid Withdrawal with Abrupt Discontinuation:If treatment is temporarily interrupted or discontinued, monitor patients for withdrawal and treat appropriately. ( 5.7)
- Risk of Hepatitis, Hepatic Events:Monitor liver function tests prior to initiation and during treatment and evaluate suspected hepatic events. ( 5.8)
- Precipitation of Opioid Withdrawal Signs and Symptoms:An opioid withdrawal syndrome is likely to occur with parenteral misuse of SUBOXONE sublingual film by individuals physically dependent on full opioid agonists, or by sublingual or buccal administration before the agonist effects of other opioids have subsided. ( 5.10)
- Risk of Overdose in Opioid‐Naïve Patients: SUBOXONE sublingual film is not appropriate as an analgesic. There have been reported deaths of opioid naïve individuals who received a 2 mg sublingual dose. ( 5.11)
ADVERSE REACTIONS
Adverse events commonly observed with the sublingual/buccal administration of the SUBOXONE sublingual film are oral hypoesthesia, glossodynia, oral mucosal erythema, headache, nausea, vomiting, hyperhidrosis, constipation, signs and symptoms of withdrawal, insomnia, pain, and peripheral edema. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Indivior Inc. at 1-877-782-6966 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Benzodiazepines: Use caution in prescribing SUBOXONE sublingual film for patients receiving benzodiazepines or other CNS depressants and warn patients against concomitant self-administration/misuse. ( 7)
- CYP3A4Inhibitors and Inducers: Monitor patients starting or ending CYP3A4 inhibitors or inducers for potential over- or under- dosing. ( 7)
- Antiretrovirals: Patients who are on chronic buprenorphine treatment should have their dose monitored if NNRTIs are added to their treatment regimen. Monitor patients taking buprenorphine and atazanavir with and without ritonavir. Dose reduction of buprenorphine may be warranted ( 7).
- Serotonergic Drugs: Concomitant use may result in serotonin syndrome. Discontinue SUBOXONE sublingual film if serotonin syndrome is suspected. ( 7)
USE IN SPECIFIC POPULATIONS
- Lactation: Buprenorphine passes into mother's milk. ( 8.2)
- Geriatric Patients: Monitor for sedation and respiratory depression. ( 8.5)
- Moderate or Severe Hepatic Impairment: Buprenorphine/naloxone products are not recommended in patients with severe hepatic impairment and may not be appropriate for patients with moderate hepatic impairment. ( 8.6)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 12/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Information
2.2 Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose
2.3 Induction
2.4 Maintenance
2.5 Method of Administration
2.6 Clinical Supervision
2.7 Unstable Patients
2.8 Discontinuing Treatment
2.9 Switching Between Buprenorphine or Buprenorphine and Naloxone Sublingual Tablets and SUBOXONE Sublingual Film
2.10 Switching Between SUBOXONE Sublingual Film Strengths
2.11 Switching Between Sublingual and Buccal Sites of Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Addiction, Abuse, and Misuse
5.2 Risk of Life-Threatening Respiratory and Central Nervous System (CNS) Depression
5.3 Managing Risks from Concomitant Use of Benzodiazepines or Other CNS Depressants
5.4 Unintentional Pediatric Exposure
5.5 Neonatal Opioid Withdrawal Syndrome
5.6 Adrenal Insufficiency
5.7 Risk of Opioid Withdrawal with Abrupt Discontinuation
5.8 Risk of Hepatitis, Hepatic Events
5.9 Hypersensitivity Reactions
5.10 Precipitation of Opioid Withdrawal Signs and Symptoms
5.11 Risk of Overdose in Opioid Naïve Patients
5.12 Use in Patients With Impaired Hepatic Function
5.13 Dental Adverse Events
5.14 QTc Prolongation
5.15 Impairment of Ability to Drive or Operate Machinery
5.16 Orthostatic Hypotension
5.17 Elevation of Cerebrospinal Fluid Pressure
5.18 Elevation of Intracholedochal Pressure
5.19 Effects in Acute Abdominal Conditions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED / STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
Storage and Disposal
Safe Use
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Information
SUBOXONE sublingual film is administered sublingually or buccally as a single daily dose.
Medication should be prescribed in consideration of the frequency of visits. Provision of multiple refills is not advised early in treatment or without appropriate patient follow‐up visits.
2.2 Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose
Discuss the availability of naloxone for the emergency treatment of opioid overdose with the patient and caregiver. Because patients being treated for opioid use disorder have the potential for relapse, putting them at risk for opioid overdose, strongly consider prescribing naloxone for the emergency treatment of opioid overdose, both when initiating and renewing treatment with SUBOXONE sublingual film. Also consider prescribing naloxone if the patient has household members (including children) or other close contacts at risk for accidental ingestion or opioid overdose [see Warnings and Precautions ( 5.2)] .
Advise patients and caregivers that naloxone may also be administered for a known or suspected overdose with SUBOXONE sublingual film itself. Higher than normal doses and repeated administration of naloxone may be necessary due to the long duration of action of SUBOXONE sublingual film and its affinity for the mu-opioid receptor [see Overdosage ( 10)] .
Inform patients and caregivers of their options for obtaining naloxone as permitted by individual state naloxone dispensing and prescribing requirements or guidelines (e.g., by prescription, directly from a pharmacist, or as part of a community-based program) [see Patient Counseling Information ( 17)] .
2.3 Induction
Prior to induction, consideration should be given to the type of opioid dependence (i.e., long‐ or short‐acting opioid products), the time since last opioid use, and the degree or level of opioid dependence.
Patients dependent on heroin or other short‐acting opioid products
Patients dependent on heroin or other short‐acting opioid products may be inducted with either SUBOXONE sublingual film or with sublingual buprenorphine monotherapy. At treatment initiation, the first dose of SUBOXONE sublingual film should be administered when objective signs of moderate opioid withdrawal appear, not less than six hours after the patient last used opioids.
It is recommended that an adequate treatment dose, titrated to clinical effectiveness, be achieved as rapidly as possible. In some studies, a too‐gradual induction over several days led to a high rate of drop‐out of buprenorphine patients during the induction period.
On Day 1, an induction dosage of up to 8 mg/2 mg SUBOXONE sublingual film is recommended. Clinicians should start with an initial dose of 2 mg/0.5 mg or 4 mg/1 mg buprenorphine/naloxone and may titrate upwards in 2 or 4 mg increments of buprenorphine, at approximately 2‐hour intervals, under supervision, to 8 mg/2 mg buprenorphine/naloxone based on the control of acute withdrawal symptoms.
On Day 2, a single daily dose of up to 16 mg/4 mg SUBOXONE sublingual film is recommended.
Because the exposure to naloxone is somewhat higher after buccal than after sublingual administration, it is recommended that the sublingual site of administration be used during induction to minimize exposure to naloxone, to reduce the risk of precipitated withdrawal.
Patients dependent on methadone or long‐acting opioid products
Patients dependent upon methadone or long‐acting opioid products may be more susceptible to precipitated and prolonged withdrawal during induction than those on short‐acting opioid products.
Buprenorphine/naloxone combination products have not been evaluated in adequate and well‐controlled studies for induction in patients who are physically dependent on long‐acting opioid products, and the naloxone in these combination products is absorbed in small amounts by the sublingual route and could cause worse precipitated and prolonged withdrawal. For this reason, buprenorphine monotherapy is recommended in patients taking long‐acting opioids when used according to approved administration instructions. Following induction, the patient may then be transitioned to once‐daily SUBOXONE sublingual film.
2.4 Maintenance
- For maintenance, SUBOXONE sublingual film may be administered buccally or sublingually.
- The dosage of SUBOXONE sublingual film from Day 3 onwards should be progressively adjusted in increments/decrements of 2 mg/0.5 mg or 4 mg/1 mg buprenorphine/naloxone to a level that holds the patient in treatment and suppresses opioid withdrawal signs and symptoms.
- After treatment induction and stabilization, the maintenance dose of SUBOXONE sublingual film is generally in the range of 4 mg/1 mg buprenorphine/naloxone to 24 mg/6 mg buprenorphine/naloxone per day depending on the individual patient and clinical response. The recommended target dosage of SUBOXONE sublingual film during maintenance is 16 mg/4 mg buprenorphine/naloxone/day as a single daily dose. Dosages higher than 24 mg/6 mg daily have not been demonstrated to provide a clinical advantage.
- When determining the prescription quantity for unsupervised administration, consider the patient's level of stability, the security of his or her home situation, and other factors likely to affect the ability to manage supplies of take‐home medication.
- There is no maximum recommended duration of maintenance treatment. Patients may require treatment indefinitely and should continue for as long as patients are benefiting and the use of SUBOXONE sublingual film contributes to the intended treatment goals.
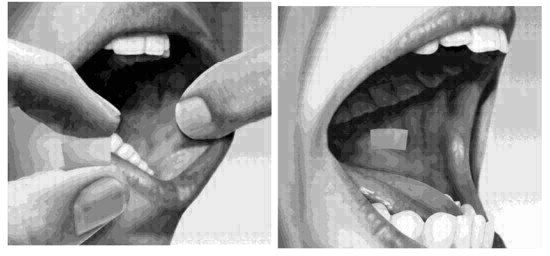
2.5 Method of Administration
SUBOXONE sublingual film must be administered whole. Do not cut, chew, or swallow SUBOXONE sublingual film. Advise patients not to eat or drink anything until the film is completely dissolved.
Sublingual Administration
Place one film under the tongue, close to the base on the left or right side. If an additional film is necessary to achieve the prescribed dose, place an additional film sublingually on the opposite side from the first film. Place the film in a manner to minimize overlapping as much as possible. The film must be kept under the tongue until the film is completely dissolved. If a third film is necessary to achieve the prescribed dose, place it under the tongue on either side after the first 2 films have dissolved.
Buccal Administration
Place one film on the inside of the right or left cheek. If an additional film is necessary to achieve the prescribed dose, place an additional film on the inside of the opposite cheek. The film must be kept on the inside of the cheek until the film is completely dissolved. If a third film is necessary to achieve the prescribed dose, place it on the inside of the right or left cheek after the first two films have dissolved.
SUBOXONE sublingual film should NOT be moved after placement.
To ensure consistency in bioavailability, patients should follow the same manner of dosing with continued use of the product. Proper administration technique should be demonstrated to the patient.
Advise patients to do the following after the product has completely dissolved in the oral mucosa: take a sip of water, swish gently around the teeth and gums, and swallow. Advise patients to wait for at least one hour after taking SUBOXONE before brushing teeth [see Warnings and Precautions ( 5.13), Postmarketing Experience ( 6.2), Information for Patients ( 17), and the Medication Guide] .
2.6 Clinical Supervision
Treatment should be initiated with supervised administration, progressing to unsupervised administration as the patient's clinical stability permits. SUBOXONE sublingual film is subject to diversion and abuse. When determining the prescription quantity for unsupervised administration, consider the patient's level of stability, the security of his or her home situation, and other factors likely to affect the ability to manage supplies of take‐home medication.
Ideally patients should be seen at reasonable intervals (e.g., at least weekly during the first month of treatment) based upon the individual circumstances of the patient. Medication should be prescribed in consideration of the frequency of visits. Provision of multiple refills is not advised early in treatment or without appropriate patient follow‐up visits. Periodic assessment is necessary to determine compliance with the dosing regimen, effectiveness of the treatment plan, and overall patient progress.
Once a stable dosage has been achieved and patient assessment (e.g., urine drug screening) does not indicate illicit drug use, less frequent follow‐up visits may be appropriate. A once‐monthly visit schedule may be reasonable for patients on a stable dosage of medication who are making progress toward their treatment objectives. Continuation or modification of pharmacotherapy should be based on the healthcare provider's evaluation of treatment outcomes and objectives such as:
- Absence of medication toxicity.
- Absence of medical or behavioral adverse effects.
- Responsible handling of medications by the patient.
- Patient's compliance with all elements of the treatment plan (including recovery‐oriented activities, psychotherapy, and/or other psychosocial modalities).
- Abstinence from illicit drug use (including problematic alcohol and/or benzodiazepine use).
If treatment goals are not being achieved, the healthcare provider should re‐evaluate the appropriateness of continuing the current treatment.
2.7 Unstable Patients
Healthcare providers will need to decide when they cannot appropriately provide further management for particular patients. For example, some patients may be abusing or dependent on various drugs, or unresponsive to psychosocial intervention such that the healthcare provider does not feel that he/she has the expertise to manage the patient. In such cases, the healthcare provider may want to assess whether to refer the patient to a specialist or more intensive behavioral treatment environment. Decisions should be based on a treatment plan established and agreed upon with the patient at the beginning of treatment.
Patients who continue to misuse, abuse, or divert buprenorphine products or other opioids should be provided with, or referred to, more intensive and structured treatment.
2.8 Discontinuing Treatment
The decision to discontinue therapy with SUBOXONE sublingual film after a period of maintenance should be made as part of a comprehensive treatment plan. Advise patients of the potential to relapse to illicit drug use following discontinuation of opioid agonist/partial agonist medication‐assisted treatment. Taper patients to reduce the occurrence of opioid withdrawal signs and symptoms [See Warnings and Precautions ( 5.7)] .
2.9 Switching Between Buprenorphine or Buprenorphine and Naloxone Sublingual Tablets and SUBOXONE Sublingual Film
Patients being switched between buprenorphine and naloxone or buprenorphine only sublingual tablets and SUBOXONE sublingual film should be started on the same dosage of the previously administered product. However, dosage adjustments may be necessary when switching between buprenorphine products. Not all strengths and combinations of the SUBOXONE sublingual films are bioequivalent to SUBOXONE® sublingual tablets as observed in pharmacokinetic studies [see Clinical Pharmacology ( 12.3)] . Therefore, systemic exposures of buprenorphine and naloxone may be different when patients are switched from tablets to film or vice‐versa. Patients should be monitored for symptoms related to over‐dosing or under‐dosing.
2.10 Switching Between SUBOXONE Sublingual Film Strengths
As indicated in Table 1, the sizes and the compositions of the four units of SUBOXONE sublingual films, i.e., 2 mg/0.5 mg, 4 mg/1 mg, 8 mg/2 mg and the 12 mg/3 mg units, are different from one another. If patients switch between various combinations of lower and higher strength units of SUBOXONE sublingual films to obtain the same total dose, (e.g., from three 4 mg/1 mg units to a single 12 mg/3 mg unit, or vice‐versa), systemic exposures of buprenorphine and naloxone may be different and patients should be monitored for over‐dosing or under‐dosing. For this reason, pharmacist should not substitute one or more film strengths for another without approval of the prescriber.
Table 1. Comparison of Available SUBOXONE Sublingual Film Strengths by Dimensions and Drug Concentrations. SUBOXONE sublingual film unit strength (buprenorphine/naloxone) SUBOXONE sublingual film unit dimensions Buprenorphine Concentration
% (w/w)Naloxone Concentration
% (w/w)2 mg/0.5 mg 22.0 mm x 12.8 mm 5.4 1.53 4 mg/1 mg
(2 times the length of the 2 mg/0.5 mg unit)22.0 mm x 25.6 mm 5.4 1.53 8 mg/2 mg 22.0 mm x 12.8 mm 17.2 4.88 12 mg/3 mg
(1.5 times the length of the 8 mg/2 mg unit)22.0 mm X 19.2 mm 17.2 4.88 2.11 Switching Between Sublingual and Buccal Sites of Administration
The systemic exposure of buprenorphine between buccal and sublingual administration of SUBOXONE sublingual film is similar. Therefore, once induction is complete, patients can switch between buccal and sublingual administration without significant risk of under or overdosing.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
SUBOXONE sublingual film is contraindicated in patients with a history of hypersensitivity to buprenorphine or naloxone as serious adverse reactions, including anaphylactic shock, have been reported [see Warnings and Precautions ( 5.9)] .
-
5 WARNINGS AND PRECAUTIONS
5.1 Addiction, Abuse, and Misuse
SUBOXONE sublingual film contains buprenorphine, a schedule III controlled substance that can be abused in a manner similar to other opioids, legal or illicit. Prescribe and dispense buprenorphine with appropriate precautions to minimize risk of misuse, abuse, or diversion, and ensure appropriate protection from theft, including in the home. Clinical monitoring appropriate to the patient's level of stability is essential. Multiple refills should not be prescribed early in treatment or without appropriate patient follow‐up visits [see Drug Abuse and Dependence ( 9.2)] .
5.2 Risk of Life-Threatening Respiratory and Central Nervous System (CNS) Depression
Buprenorphine has been associated with life‐threatening respiratory depression and death. Many, but not all, post‐marketing reports regarding coma and death involved misuse by self‐injection or were associated with the concomitant use of buprenorphine and benzodiazepines or other CNS depressants, including alcohol. Warn patients of the potential danger of self‐administration of benzodiazepines or other CNS depressants while under treatment with SUBOXONE sublingual film [see Warnings and Precautions ( 5.3), Drug Interactions ( 7)].
Use SUBOXONE sublingual film with caution in patients with compromised respiratory function (e.g., chronic obstructive pulmonary disease, cor pulmonale, decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression).
Educate patients and caregivers on how to recognize respiratory depression and emphasize the importance of calling 911 or getting emergency medical help right away in the event of a known or suspected overdose [see Patient Counseling Information ( 17)] .
Opioids can cause sleep-related breathing disorders including central sleep apnea (CSA) and sleep-related hypoxemia. Opioid use increases the risk of CSA in a dose-dependent fashion. In patients who present with CSA, consider decreasing the opioid dosage using best practices for opioid taper [see Dosage and Administration ( 2.8)] .
Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose
Discuss the availability of naloxone for the emergency treatment of opioid overdose with the patient and caregiver.
Because patients being treated for opioid use disorder have the potential for relapse, putting them at risk for opioid overdose, strongly consider prescribing naloxone for the emergency treatment of opioid overdose, both when initiating and renewing treatment with SUBOXONE sublingual film. Also consider prescribing naloxone if the patient has household members (including children) or other close contacts at risk for accidental ingestion or opioid overdose [see Dosage and Administration ( 2.2)] .
Advise patients and caregivers that naloxone may also be administered for a known or suspected overdose with SUBOXONE sublingual film itself. Higher than normal doses and repeated administration of naloxone may be necessary due to the long duration of action of SUBOXONE sublingual film and its affinity for the mu-opioid receptor [see Overdosage ( 10)] .
Inform patients and caregivers of their options for obtaining naloxone as permitted by individual state naloxone dispensing and prescribing requirements or guidelines (e.g., by prescription, directly from a pharmacist, or as part of a community-based program).
Educate patients and caregivers on how to recognize respiratory depression and, if naloxone is prescribed, how to treat with naloxone. Emphasize the importance of calling 911 or getting emergency medical help, even if naloxone is administered [see Patient Counseling Information ( 17)] .
5.3 Managing Risks from Concomitant Use of Benzodiazepines or Other CNS Depressants
Concomitant use of buprenorphine and benzodiazepines or other CNS depressants increases the risk of adverse reactions including overdose and death. Medication‐assisted treatment of opioid use disorder, however, should not be categorically denied to patients taking these drugs. Prohibiting or creating barriers to treatment can pose an even greater risk of morbidity and mortality due to the opioid use disorder alone.
As a routine part of orientation to buprenorphine treatment, educate patients about the risks of concomitant use of benzodiazepines, sedatives, opioid analgesics, and alcohol.
Develop strategies to manage use of prescribed or illicit benzodiazepines or other CNS depressants at initiation of buprenorphine treatment, or if it emerges as a concern during treatment. Adjustments to induction procedures and additional monitoring may be required. There is no evidence to support dose limitations or arbitrary caps of buprenorphine as a strategy to address benzodiazepine use in buprenorphine‐treated patients. However, if a patient is sedated at the time of buprenorphine dosing, delay or omit the buprenorphine dose if appropriate.
Cessation of benzodiazepines or other CNS depressants is preferred in most cases of concomitant use. In some cases, monitoring in a higher level of care for taper may be appropriate. In others, gradually tapering a patient off of a prescribed benzodiazepine or other CNS depressant or decreasing to the lowest effective dose may be appropriate.
For patients in buprenorphine treatment, benzodiazepines are not the treatment of choice for anxiety or insomnia. Before co‐prescribing benzodiazepines, ensure that patients are appropriately diagnosed and consider alternative medications and non‐pharmacologic treatments to address anxiety or insomnia. Ensure that other healthcare providers prescribing benzodiazepines or other CNS depressants are aware of the patient's buprenorphine treatment and coordinate care to minimize the risks associated with concomitant use.
If concomitant use is warranted, strongly consider prescribing naloxone for the emergency treatment of opioid overdose, as is recommended for all patients in buprenorphine treatment for opioid use disorder [see Warnings and Precautions ( 5.2)].
In addition, take measures to confirm that patients are taking their medications as prescribed and are not diverting or supplementing with illicit drugs. Toxicology screening should test for prescribed and illicit benzodiazepines [see Drug Interactions ( 7)].
5.4 Unintentional Pediatric Exposure
Buprenorphine can cause severe, possibly fatal, respiratory depression in children who are accidentally exposed to it. Store buprenorphine‐containing medications safely out of the sight and reach of children and destroy any unused medication appropriately [see Patient Counseling Information ( 17) ].
5.5 Neonatal Opioid Withdrawal Syndrome
Neonatal opioid withdrawal syndrome (NOWS) is an expected and treatable outcome of prolonged use of opioids during pregnancy, whether that use is medically‐authorized or illicit. Unlike opioid withdrawal syndrome in adults, NOWS may be life‐threatening if not recognized and treated in the neonate. Healthcare professionals should observe newborns for signs of NOWS and manage accordingly [see Use in Specific Populations ( 8.1)] .
Advise pregnant women receiving opioid addiction treatment with SUBOXONE sublingual film of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available [see Use in Specific Populations ( 8.1)] . This risk must be balanced against the risk of untreated opioid addiction which often results in continued or relapsing illicit opioid use and is associated with poor pregnancy outcomes. Therefore, prescribers should discuss the importance and benefits of management of opioid addiction throughout pregnancy.
5.6 Adrenal Insufficiency
Cases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use. Presentation of adrenal insufficiency may include non‐specific symptoms and signs including nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. If adrenal insufficiency is suspected, confirm the diagnosis with diagnostic testing as soon as possible. If adrenal insufficiency is diagnosed, treat with physiologic replacement doses of corticosteroids. Wean the patient off of the opioid to allow adrenal function to recover and continue corticosteroid treatment until adrenal function recovers. Other opioids may be tried as some cases reported use of a different opioid without recurrence of adrenal insufficiency. The information available does not identify any particular opioids as being more likely to be associated with adrenal insufficiency.
5.7 Risk of Opioid Withdrawal with Abrupt Discontinuation
Buprenorphine is a partial agonist at the mu‐opioid receptor and chronic administration produces physical dependence of the opioid type, characterized by withdrawal signs and symptoms upon abrupt discontinuation or rapid taper. The withdrawal syndrome is typically milder than seen with full agonists and may be delayed in onset [see Drug Abuse and Dependence ( 9.3)] . When discontinuing SUBOXONE sublingual film, gradually taper the dosage [see Dosage and Administration ( 2.8)] .
5.8 Risk of Hepatitis, Hepatic Events
Cases of cytolytic hepatitis and hepatitis with jaundice have been observed in individuals receiving buprenorphine in clinical trials and through post‐marketing adverse event reports. The spectrum of abnormalities ranges from transient asymptomatic elevations in hepatic transaminases to case reports of death, hepatic failure, hepatic necrosis, hepatorenal syndrome, and hepatic encephalopathy. In many cases, the presence of pre‐existing liver enzyme abnormalities, infection with hepatitis B or hepatitis C virus, concomitant usage of other potentially hepatotoxic drugs, and ongoing injecting drug use may have played a causative or contributory role. In other cases, insufficient data were available to determine the etiology of the abnormality. Withdrawal of buprenorphine has resulted in amelioration of acute hepatitis in some cases;
however, in other cases no dose reduction was necessary. The possibility exists that buprenorphine had a causative or contributory role in the development of the hepatic abnormality in some cases. Liver function tests, prior to initiation of treatment, are recommended to establish a baseline. Periodic monitoring of liver function during treatment is also recommended. A biological and etiological evaluation is recommended when a hepatic event is suspected. Depending on the case, SUBOXONE sublingual film may need to be carefully discontinued to prevent withdrawal signs and symptoms and a return by the patient to illicit drug use, and strict monitoring of the patient should be initiated.
5.9 Hypersensitivity Reactions
Cases of hypersensitivity to buprenorphine and naloxone containing products have been reported both in clinical trials and in the post‐marketing experience. Cases of bronchospasm, angioneurotic edema, and anaphylactic shock have been reported. The most common signs and symptoms include rashes, hives, and pruritus. A history of hypersensitivity to buprenorphine or naloxone is a contraindication to the use of SUBOXONE sublingual film.
5.10 Precipitation of Opioid Withdrawal Signs and Symptoms
Because it contains naloxone, SUBOXONE sublingual film is likely to produce withdrawal signs and symptoms if misused parenterally by individuals dependent on full opioid agonists such as heroin, morphine, or methadone. Because of the partial agonist properties of buprenorphine, SUBOXONE sublingual film may precipitate opioid withdrawal signs and symptoms in such persons if administered before the agonist effects of the opioid have subsided.
5.11 Risk of Overdose in Opioid Naïve Patients
There have been reported deaths of opioid ‐naïve individuals who received a 2 mg dose of buprenorphine as a sublingual tablet for analgesia. SUBOXONE sublingual film is not appropriate as an analgesic.
5.12 Use in Patients With Impaired Hepatic Function
Buprenorphine/naloxone products are not recommended in patients with severe hepatic impairment and may not be appropriate for patients with moderate hepatic impairment. The doses of buprenorphine and naloxone in this fixed‐dose combination product cannot be individually titrated, and hepatic impairment results in a reduced clearance of naloxone to a much greater extent than buprenorphine. Therefore, patients with severe hepatic impairment will be exposed to substantially higher levels of naloxone than patients with normal hepatic function. This may result in an increased risk of precipitated withdrawal at the beginning of treatment (induction) and may interfere with buprenorphine's efficacy throughout treatment. In patients with moderate hepatic impairment, the differential reduction of naloxone clearance compared to buprenorphine clearance is not as great as in subjects with severe hepatic impairment. However, buprenorphine/naloxone products are not recommended for initiation of treatment (induction) in patients with moderate hepatic impairment due to the increased risk of precipitated withdrawal. Buprenorphine/naloxone products may be used with caution for maintenance treatment in patients with moderate hepatic impairment who have initiated treatment on a buprenorphine product without naloxone. However, patients should be carefully monitored and consideration given to the possibility of naloxone interfering with buprenorphine's efficacy [see Use in Specific Populations ( 8.6)].
5.13 Dental Adverse Events
Cases of dental caries, some severe (i.e., tooth fracture, tooth loss), have been reported following the use of transmucosal buprenorphine-containing products. Reported events include cavities, tooth decay, dental abscesses/infection, rampant caries, tooth erosion, fillings falling out, and, in some cases, total tooth loss. Treatment for these events included tooth extraction, root canal, dental surgery, as well as other restorative procedures (i.e., fillings, crowns, implants, dentures). Multiple cases were reported in individuals without any prior history of dental problems.
Refer patients to dental care services and encourage them to have regular dental checkups while taking SUBOXONE. Educate patients to seek dental care and strategies to maintain or improve oral health while being treated with transmucosal buprenorphine-containing products. Strategies include, but are not limited to, gently rinsing the teeth and gums with water and then swallowing after SUBOXONE has been completely dissolved in the oral mucosa. Advise patients to wait for at least one hour after taking SUBOXONE before brushing teeth [see Dosing and Administration ( 2.5), Information for Patients ( 17), Medication Guide] .
5.14 QTc Prolongation
Thorough QT studies with buprenorphine products have demonstrated QT prolongation ≤ 15 msec. This QTc prolongation effect does not appear to be mediated by hERG channels. Based on these two findings, buprenorphine is unlikely to be pro-arrhythmic when used alone in patients without risk factors. The risk of combining buprenorphine with other QT-prolonging agents is not known.
Consider these observations in clinical decisions when prescribing SUBOXONE sublingual film to patients with risk factors such as hypokalemia, bradycardia, recent conversion from atrial fibrillation, congestive heart failure, digitalis therapy, baseline QT prolongation, subclinical long-QT syndrome, or severe hypomagnesemia.
5.15 Impairment of Ability to Drive or Operate Machinery
SUBOXONE sublingual film may impair the mental or physical abilities required for the performance of potentially dangerous tasks such as driving a car or operating machinery, especially during treatment induction and dose adjustment. Caution patients about driving or operating hazardous machinery until they are reasonably certain that SUBOXONE sublingual film therapy does not adversely affect his or her ability to engage in such activities.
5.16 Orthostatic Hypotension
Like other opioids, SUBOXONE sublingual film may produce orthostatic hypotension in ambulatory patients.
5.17 Elevation of Cerebrospinal Fluid Pressure
Buprenorphine, like other opioids, may elevate cerebrospinal fluid pressure and should be used with caution in patients with head injury, intracranial lesions, and other circumstances when cerebrospinal pressure may be increased. Buprenorphine can produce miosis and changes in the level of consciousness that may interfere with patient evaluation.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Addiction, Abuse, and Misuse [ see Warnings and Precautions ( 5.1) ]
- Respiratory and CNS Depression [see Warnings and Precautions ( 5.2), ( 5.3)]
- Neonatal Opioid Withdrawal Syndrome [see Warnings and Precautions ( 5.5)]
- Adrenal Insufficiency [see Warnings and Precautions ( 5.6)]
- Opioid Withdrawal [see Warnings and Precautions ( 5.7, 5.10)]
- Hepatitis, Hepatic Events [see Warnings and Precautions ( 5.8)]
- Hypersensitivity Reactions [see Warnings and Precautions ( 5.9)]
- Orthostatic Hypotension [see Warnings and Precautions ( 5.16)]
- Elevation of Cerebrospinal Fluid Pressure [see Warnings and Precautions ( 5.17)]
- Elevation of Intracholedochal Pressure [see Warnings and Precautions ( 5.18)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of SUBOXONE sublingual film is supported by clinical trials using SUBUTEX® (buprenorphine) sublingual tablets and SUBOXONE (buprenorphine and naloxone) sublingual tablets, and other trials using buprenorphine sublingual solutions, as well as an open-label study in 194 patients treated with SUBOXONE sublingual film administered sublingually and 188 patients treated with the film administered buccally. In total, safety data from clinical studies are available from over 3000 opioid-dependent subjects exposed to buprenorphine at doses in the range used in the treatment of opioid dependence. Few differences in the adverse event profile were noted with regard to sublingually and bucally administered SUBOXONE sublingual film, SUBOXONE sublingual tablets, SUBUTEX sublingual tablets and a buprenorphine ethanolic sublingual solution.
The most common adverse event (> 1%) associated with the sublingual administration of the SUBOXONE sublingual film was oral hypoesthesia. Other adverse events were constipation, glossodynia, oral mucosal erythema, vomiting, intoxication, disturbance in attention, palpitations, insomnia, withdrawal syndrome, hyperhidrosis, and blurred vision.
The most common adverse events associated with the buccal administration were similar to those observed with sublingual administration of the film.
Other adverse event data were derived from larger, controlled studies of SUBOXONE sublingual tablets and SUBUTEX sublingual tablets and of buprenorphine sublingual solution. In a comparative study of SUBOXONE sublingual tablets and SUBUTEX sublingual tablets, adverse event profiles were similar for subjects treated with 16 mg/4 mg SUBOXONE sublingual tablets or 16 mg SUBUTEX sublingual tablets. The following adverse events were reported to occur by at least 5% of patients in a 4 week study of SUBOXONE sublingual tablets and SUBUTEX sublingual tablets.
Table 2. Adverse Events (≥ 5%) by Body System and Treatment Group in a 4 Week Study Abbreviations: COSTART = Coding Symbols for Thesaurus of Adverse Reaction Terms.
Body System/ Adverse Event (COSTART Terminology)SUBOXONE sublingual tablets
16 mg/4 mg/day N = 107
n (%)SUBUTEX sublingual tablets
16 mg/day N = 103
n (%)
Placebo N = 107 n (%)Body as a Whole Asthenia 7 (6.5%) 5 (4.9%) 7 (6.5%) Chills 8 (7.5%) 8 (7.8%) 8 (7.5%) Headache 39 (36.4%) 30 (29.1%) 24 (22.4%) Infection 6 (5.6%) 12 (11.7%) 7 (6.5%) Pain 24 (22.4%) 19 (18.4%) 20 (18.7%) Pain abdomen 12 (11.2%) 12 (11.7%) 7 (6.5%) Pain back 4 (3.7%) 8 (7.8%) 12 (11.2%) Withdrawal syndrome 27 (25.2%) 19 (18.4%) 40 (37.4%) Cardiovascular System Vasodilation 10 (9.3%) 4 (3.9%) 7 (6.5%) Digestive System Constipation 13 (12.1%) 8 (7.8%) 3 (2.8%) Diarrhea 4 (3.7%) 5 (4.9%) 16 (15.0%) Nausea 16 (15.0%) 14 (13.6%) 12 (11.2%) Vomiting 8 (7.5%) 8 (7.8%) 5 (4.7%) Nervous System Insomnia 15 (14.0%) 22 (21.4%) 17 (15.9%) Respiratory System Rhinitis 5 (4.7%) 10 (9.7%) 14 (13.1%) Skin And Appendages Sweating 15 (14.0%) 13 (12.6%) 11 (10.3%) The adverse event profile of buprenorphine was also characterized in the dose-controlled study of a buprenorphine ethanolic solution, over a range of doses in four months of treatment. Table 3shows adverse events reported by at least 5% of subjects in any dose group in the dose-controlled trial.
Table 3. Adverse Events (≥ 5%) by Body System and Treatment Group in a 16 Week Study *Sublingual solution. Doses in this table cannot necessarily be delivered in tablet form, but for comparison purposes:
"Very low" dose (1 mg solution) would be less than a tablet dose of 2 mg
"Low" dose (4 mg solution) approximates a 6 mg tablet dose
"Moderate" dose (8 mg solution) approximates a 12 mg tablet dose
"High" dose (16 mg solution) approximates a 24 mg tablet dose
Body System/ Adverse Event
(COSTART
Terminology)Buprenorphine Dose Very Low* N = 184
n (%)Low*
N = 180 n (%)Moderate* N = 186
n (%)High*
N = 181 n (%)Total*
N = 731 n (%)Body as a Whole Abscess 9 (5%) 2 (1%) 3 (2%) 2 (1%) 16 (2%) Asthenia 26 (14%) 28 (16%) 26 (14%) 24 (13%) 104 (14%) Chills 11 (6%) 12 (7%) 9 (5%) 10 (6%) 42 (6%) Fever 7 (4%) 2 (1%) 2 (1%) 10 (6%) 21 (3%) Flu syndrome 4 (2%) 13 (7%) 19 (10%) 8 (4%) 44 (6%) Headache 51 (28%) 62 (34%) 54 (29%) 53 (29%) 220 (30%) Infection 32 (17%) 39 (22%) 38 (20%) 40 (22%) 149 (20%) Injury accidental 5 (3%) 10 (6%) 5 (3%) 5 (3%) 25 (3%) Pain 47 (26%) 37 (21%) 49 (26%) 44 (24%) 177 (24%) Pain back 18 (10%) 29 (16%) 28 (15%) 27 (15%) 102 (14%) Withdrawal syndrome 45 (24%) 40 (22%) 41 (22%) 36 (20%) 162 (22%) Digestive System Constipation 10 (5%) 23 (13%) 23 (12%) 26 (14%) 82 (11%) Diarrhea 19 (10%) 8 (4%) 9 (5%) 4 (2%) 40 (5%) Dyspepsia 6 (3%) 10 (6%) 4 (2%) 4 (2%) 24 (3%) Nausea 12 (7%) 22 (12%) 23 (12%) 18 (10%) 75 (10%) Vomiting 8 (4%) 6 (3%) 10 (5%) 14 (8%) 38 (5%) Nervous System Anxiety 22 (12%) 24 (13%) 20 (11%) 25 (14%) 91 (12%) Depression 24 (13%) 16 (9%) 25 (13%) 18 (10%) 83 (11%) Dizziness 4 (2%) 9 (5%) 7 (4%) 11 (6%) 31 (4%) Insomnia 42 (23%) 50 (28%) 43 (23%) 51 (28%) 186 (25%) Nervousness 12 (7%) 11 (6%) 10 (5%) 13 (7%) 46 (6%) Somnolence 5 (3%) 13 (7%) 9 (5%) 11 (6%) 38 (5%) Respiratory System Cough increase 5 (3%) 11 (6%) 6 (3%) 4 (2%) 26 (4%) Pharyngitis 6 (3%) 7 (4%) 6 (3%) 9 (5%) 28 (4%) Rhinitis 27 (15%) 16 (9%) 15 (8%) 21 (12%) 79 (11%) Skin and Appendages Sweat 23 (13%) 21 (12%) 20 (11%) 23 (13%) 87 (12%) Special Senses Runny eyes 13 (7%) 9 (5%) 6 (3%) 6 (3%) 34 (5%) The safety of SUBOXONE sublingual film during treatment induction is supported by a clinical trial using 16 patients treated with SUBOXONE sublingual film and 18 treated with a buprenorphine-only sublingual film. Few differences in the adverse event profiles were noted between SUBOXONE sublingual film and the buprenorphine-only sublingual film.
The most common adverse event occurring during treatment induction and the 3 days following induction using SUBOXONE sublingual film was restlessness. Other adverse events were anxiety, piloerection, stomach discomfort, irritability, headache, rhinorrhea, cold sweat, arthralgia, and lacrimation increased.
Four subjects left the study early on the first day of sublingual film administration. However, there was no evidence to suggest that any of the four subjects experienced precipitated withdrawal secondary to the administration of buprenorphine or buprenorphine/naloxone sublingual films.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of SUBOXONE sublingual film. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The most frequently reported postmarketing adverse events were peripheral edema, stomatitis, glossitis, and blistering and ulceration of the mouth or tongue.
Serotonin syndrome: Cases of serotonin syndrome, a potentially life-threatening condition, have been reported during concomitant use of opioids with serotonergic drugs.
Adrenal insufficiency: Cases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use.
Anaphylaxis: Anaphylaxis has been reported with ingredients contained in SUBOXONE sublingual film.
Androgen deficiency: Cases of androgen deficiency have occurred with chronic use of opioids [see Clinical Pharmacology ( 12.2)] .
Local reactions: Dental decay (including caries, tooth fracture, and tooth loss), glossodynia, glossitis, oral mucosal erythema, oral hypoesthesia, and stomatitis
Hypoglycemia: Cases of hypoglycemia have been reported in patients taking opioids. Most reports were in patients with at least one predisposing risk factor (e.g., diabetes).
-
7 DRUG INTERACTIONS
Table 4Includes clinically significant drug interactions with SUBOXONE.
Table 4. Clinically Significant Drug Interactions Benzodiazepines and Other Central Nervous System (CNS) Depressants Clinical Impact: Due to additive pharmacologic effects, the concomitant use of benzodiazepines or other CNS depressants, including alcohol, increases the risk of respiratory depression, profound sedation, coma, and death. Intervention: Cessation of benzodiazepines or other CNS depressants is preferred in most cases of concomitant use. In some cases, monitoring in a higher level of care for taper may be appropriate. In others, gradually tapering a patient off of a prescribed benzodiazepine or other CNS depressant or decreasing to the lowest effective dose may be appropriate.
Before co-prescribing benzodiazepines for anxiety or insomnia, ensure that patients are appropriately diagnosed and consider alternative medications and non-pharmacologic treatments [see Warnings and Precautions ( 5.2, 5.3)] .
If concomitant use is warranted, strongly consider prescribing naloxone for the emergency treatment of opioid overdose, as is recommended for all patients in treatment for opioid use disorder [see Warnings and Precautions ( 5.2)] .Examples: Alcohol, benzodiazepines and other sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, and other opioids. Inhibitors of CYP3A4 Clinical Impact: The concomitant use of buprenorphine and CYP3A4 inhibitors can increase the plasma concentration of buprenorphine, resulting in increased or prolonged opioid effects, particularly when an inhibitor is added after a stable dose of SUBOXONE sublingual film is achieved.
After stopping a CYP3A4 inhibitor, as the effects of the inhibitor decline, the buprenorphine plasma concentration will decrease [see Clinical Pharmacology ( 12.3)] , potentially resulting in decreased opioid efficacy or a withdrawal syndrome in patients who had developed physical dependence to buprenorphine.Intervention: If concomitant use is necessary, consider dosage reduction of SUBOXONE sublingual film until stable drug effects are achieved. Monitor patients for respiratory depression and sedation at frequent intervals.
If a CYP3A4 inhibitor is discontinued, consider increasing the SUBOXONE sublingual film dosage until stable drug effects are achieved. Monitor for signs of opioid withdrawal.Examples: Macrolide antibiotics (e.g., erythromycin), azole-antifungal agents (e.g. ketoconazole), protease inhibitors (e.g., ritonavir) CYP3A4 Inducers Clinical Impact: The concomitant use of buprenorphine and CYP3A4 inducers can decrease the plasma concentration of buprenorphine [see Clinical Pharmacology ( 12.3)] , potentially resulting in decreased efficacy or onset of a withdrawal syndrome in patients who have developed physical dependence to buprenorphine.
After stopping a CYP3A4 inducer, as the effects of the inducer decline, the buprenorphine plasma concentration will increase [see Clinical Pharmacology ( 12.3)] , which could increase or prolong both therapeutic effects and adverse reactions and may cause serious respiratory depression.Intervention: If concomitant use is necessary, consider increasing the SUBOXONE sublingual film dosage until stable drug effects are achieved. Monitor for signs of opioid withdrawal.
If a CYP3A4 inducer is discontinued, consider SUBOXONE sublingual film dosage reduction and monitor for signs of respiratory depression.Examples: Rifampin, carbamazepine, phenytoin Antiretrovirals: Non-nucleoside reverse transcriptase inhibitors (NNRTIs) Clinical Impact: Non-nucleoside reverse transcriptase inhibitors (NNRTIs) are metabolized principally by CYP3A4. Efavirenz, nevirapine, and etravirine are known CYP3A inducers, whereas delavirdine is a CYP3A inhibitor. Significant pharmacokinetic interactions between NNRTIs (e.g., efavirenz and delavirdine) and buprenorphine have been shown in clinical studies, but these pharmacokinetic interactions did not result in any significant pharmacodynamic effects. Intervention: Patients who are on chronic SUBOXONE sublingual film treatment should have their dose monitored if NNRTIs are added to their treatment regimen. Examples: efavirenz, nevirapine, etravirine, delavirdine Antiretrovirals: Protease inhibitors (PIs) Clinical Impact: Studies have shown some antiretroviral protease inhibitors (PIs) with CYP3A4 inhibitory activity (nelfinavir, lopinavir/ritonavir, ritonavir) have little effect on buprenorphine pharmacokinetic and no significant pharmacodynamic effects. Other PIs with CYP3A4 inhibitory activity (atazanavir and atazanavir/ritonavir) resulted in elevated levels of buprenorphine and norbuprenorphine, and patients in one study reported increased sedation. Symptoms of opioid excess have been found in post-marketing reports of patients receiving buprenorphine and atazanavir with and without ritonavir concomitantly. Intervention: Monitor patients taking SUBOXONE sublingual film and atazanavir with and without ritonavir, and reduce dose of SUBOXONE sublingual film if warranted. Examples: atazanavir, ritonavir Antiretrovirals: Nucleoside reverse transcriptase inhibitors (NRTIs) Clinical Impact: Nucleoside reverse transcriptase inhibitors (NRTIs) do not appear to induce or inhibit the P450 enzyme pathway, thus no interactions with buprenorphine are expected. Intervention: None Serotonergic Drugs Clinical Impact: The concomitant use of opioids with other drugs that affect the serotonergic neurotransmitter system has resulted in serotonin syndrome. Intervention: If concomitant use is warranted, carefully observe the patient, particularly during treatment initiation and dose adjustment. Discontinue SUBOXONE sublingual film sublingual film if serotonin syndrome is suspected. Examples: Selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), triptans, 5-HT3 receptor antagonists, drugs that affect the serotonin neurotransmitter system (e.g., mirtazapine, trazodone, tramadol), certain muscle relaxants (i.e., cyclobenzaprine, metaxalone), monoamine oxidase (MAO) inhibitors (those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue). Monoamine Oxidase Inhibitors (MAOIs) Clinical Impact: MAOI interactions with opioids may manifest as serotonin syndrome or opioid toxicity (e.g., respiratory depression, coma). Intervention: The use of SUBOXONE sublingual film is not recommended for patients taking MAOIs or within 14 days of stopping such treatment. Examples: phenelzine, tranylcypromine, linezolid Muscle Relaxants Clinical Impact: Buprenorphine may enhance the neuromuscular blocking action of skeletal muscle relaxants and produce an increased degree of respiratory depression. Intervention: Monitor patients receiving muscle relaxants and SUBOXONE sublingual film for signs of respiratory depression that may be greater than otherwise expected and decrease the dosage of SUBOXONE sublingual film and/or the muscle relaxant as necessary. Due to the risk of respiratory depression with concomitant use of skeletal muscle relaxants and opioids, strongly consider prescribing naloxone for the emergency treatment of opioid overdose [see Dosage and Administration ( 2.2), Warnings and Precautions ( 5.2, 5.3)] . Diuretics Clinical Impact: Opioids can reduce the efficacy of diuretics by inducing the release of antidiuretic hormone. Intervention: Monitor patients for signs of diminished diuresis and/or effects on blood pressure and increase the dosage of the diuretic as needed. Anticholinergic Drugs Clinical Impact: The concomitant use of anticholinergic drugs may increase the risk of urinary retention and/or severe constipation, which may lead to paralytic ileus. Intervention: Monitor patients for signs of urinary retention or reduced gastric motility when SUBOXONE sublingual film is used concomitantly with anticholinergic drugs. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The data on use of buprenorphine, one of the active ingredients in SUBOXONE sublingual film, in pregnancy, are limited; however, these data do not indicate an increased risk of major malformations specifically due to buprenorphine exposure. There are limited data from randomized clinical trials in women maintained on buprenorphine that were not designed appropriately to assess the risk of major malformations [see Data]. Observational studies have reported on congenital malformations among buprenorphine-exposed pregnancies, but were also not designed appropriately to assess the risk of congenital malformations specifically due to buprenorphine exposure [ see Data]. The extremely limited data on sublingual naloxone exposure in pregnancy are not sufficient to evaluate a drug-associated risk.
Reproductive and developmental studies in rats and rabbits identified adverse events at clinically relevant and higher doses. Embryofetal death was observed in both rats and rabbits administered buprenorphine during the period of organogenesis at doses approximately 6 and 0.3 times, respectively, the human sublingual dose of 16 mg/day of buprenorphine. Pre-and postnatal development studies in rats demonstrated increased neonatal deaths at 0.3 times and above and dystocia at approximately 3 times the human sublingual dose of 16 mg/day of buprenorphine. No clear teratogenic effects were seen when buprenorphine was administered during organogenesis with a range of doses equivalent to or greater than the human sublingual dose of 16 mg/day of buprenorphine. However, increases in skeletal abnormalities were noted in rats and rabbits administered buprenorphine daily during organogenesis at doses approximately 0.6 and approximately equal to the human sublingual dose of 16 mg/day of buprenorphine, respectively. In a few studies, some events such as acephalus and omphalocele were also observed but these findings were not clearly treatment-related [see Data]. Based on animal data, advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Disease-associated maternal and embryo-fetal risk
Untreated opioid addiction in pregnancy is associated with adverse obstetrical outcomes such as low birth weight, preterm birth, and fetal death. In addition, untreated opioid addiction often results in continued or relapsing illicit opioid use.
Dose Adjustment during Pregnancy and the Postpartum Period
Dosage adjustments of buprenorphine may be required during pregnancy, even if the patient was maintained on a stable dose prior to pregnancy. Withdrawal signs and symptoms should be monitored closely and the dose adjusted as necessary.
Fetal/neonatal adverse reactions
Neonatal opioid withdrawal syndrome may occur in newborn infants of mothers who are receiving treatment with SUBOXONE sublingual film.
Neonatal opioid withdrawal syndrome presents as irritability, hyperactivity and abnormal sleep pattern, high pitched cry, tremor, vomiting, diarrhea, and/or failure to gain weight. Signs of neonatal withdrawal usually occur in the first days after birth. The duration and severity of neonatal opioid withdrawal syndrome may vary. Observe newborns for signs of neonatal opioid withdrawal syndrome and manage accordingly [see Warnings and Precautions ( 5.5)] .
Labor or Delivery
Opioid-dependent women on buprenorphine maintenance therapy may require additional analgesia during labor.
Data
Human Data
Studies have been conducted to evaluate neonatal outcomes in women exposed to buprenorphine during pregnancy. Limited data on malformations from trials, observational studies, case series, and case reports on buprenorphine use in pregnancy do not indicate an increased risk of major malformations specifically due to buprenorphine. Several factors may complicate the interpretation of investigations of the children of women who take buprenorphine during pregnancy, including maternal use of illicit drugs, late presentation for prenatal care, infection, poor compliance, poor nutrition, and psychosocial circumstances. Interpretation of data is complicated further by the lack of information on untreated opioid-dependent pregnant women, who would be the most appropriate group for comparison. Rather, women on another form of opioid medication- assisted treatment, or women in the general population are generally used as the comparison group. However, women in these comparison groups may be different from women prescribed buprenorphine-containing products with respect to maternal factors that may lead to poor pregnancy outcomes.
In a multicenter, double-blind, randomized, controlled trial [Maternal Opioid Treatment: Human Experimental Research (MOTHER)] designed primarily to assess neonatal opioid withdrawal effects, opioid-dependent pregnant women were randomized to buprenorphine (n = 86) or methadone (n = 89) treatment, with enrollment at an average gestational age of 18.7 weeks in both groups. A total of 28 of the 86 women in the buprenorphine group (33%) and 16 of the 89 women in the methadone group (18%) discontinued treatment before the end of pregnancy.
Among women who remained in treatment until delivery, there was no difference between buprenorphine- treated and methadone-treated groups in the number of neonates requiring NOWS treatment or in the peak severity of NOWS. Buprenorphine-exposed neonates required less morphine (mean total dose, 1.1 mg vs. 10.4 mg), had shorter hospital stays (10.0 days vs. 17.5 days), and shorter duration of treatment for NOWS (4.1 days vs. 9.9 days) compared to the methadone-exposed group. There were no differences between groups in other primary outcomes (neonatal head circumference,) or secondary outcomes (weight and length at birth, preterm birth, gestational age at delivery, and 1-minute and 5-minute Apgar scores), or in the rates of maternal or neonatal adverse events. The outcomes among mothers who discontinued treatment before delivery and may have relapsed to illicit opioid use are not known. Because of the imbalance in discontinuation rates between the buprenorphine and methadone groups, the study findings are difficult to interpret.
Animal Data
The exposure margins listed below are based on body surface area comparisons (mg/m 2) to the human sublingual dose of 16 mg buprenorphine via SUBOXONE sublingual tablets.
Effects on embryo-fetal development were studied in Sprague-Dawley rats and Russian white rabbits following oral (1:1) and intramuscular (IM) (3:2) administration of mixtures of buprenorphine and naloxone during the period of organogenesis. Following oral administration to rats no teratogenic effects were observed at buprenorphine doses up to 250 mg/kg/day (estimated exposure approximately 150 times the human sublingual dose of 16 mg) in the presence of maternal toxicity (mortality). Following oral administration to rabbits, no teratogenic effects were observed at buprenorphine doses up to 40 mg/kg/day (estimated exposure approximately 50 times, the human sublingual dose of 16 mg) in the absence of clear maternal toxicity.
No definitive drug-related teratogenic effects were observed in rats and rabbits at IM doses up to
30 mg/kg/day (estimated exposure approximately 20 times and 35 times, respectively, the human sublingual dose of 16 mg). Maternal toxicity resulting in mortality was noted in these studies in both rats and rabbits.
Acephalus was observed in one rabbit fetus from the low-dose group and omphalocele was observed in two rabbit fetuses from the same litter in the mid-dose group; no findings were observed in fetuses from the high-dose group. Maternal toxicity was seen in the high-dose group but not at the lower doses where the findings were observed. Following oral administration of buprenorphine to rats, dose-related post-implantation losses, evidenced by increases in the numbers of early resorptions with consequent reductions in the numbers of fetuses, were observed at doses of 10 mg/kg/day or greater (estimated exposure approximately 6 times the human sublingual dose of 16 mg). In the rabbit, increased post-implantation losses occurred at an oral dose of 40 mg/kg/day. Following IM administration in the rat and the rabbit, post-implantation losses, as evidenced by decreases in live fetuses and increases in resorptions, occurred at 30 mg/kg/day.
Buprenorphine was not teratogenic in rats or rabbits after IM or subcutaneous (SC) doses up to 5 mg/kg/day (estimated exposure was approximately 3 and 6 times, respectively, the human sublingual dose of 16 mg), after IV doses up to 0.8 mg/kg/day (estimated exposure was approximately 0.5 times and equal to, respectively, the human sublingual dose of 16 mg), or after oral doses up to 160 mg/kg/day in rats (estimated exposure was approximately 95 times the human sublingual dose of 16 mg) and 25 mg/kg/day in rabbits (estimated exposure was approximately 30 times the human daily sublingual dose of 16 mg). Significant increases in skeletal abnormalities (e.g., extra thoracic vertebra or thoraco-lumbar ribs) were noted in rats after SC administration of 1 mg/kg/day and up (estimated exposure was approximately 0.6 times the human sublingual dose of 16 mg), but were not observed at oral doses up to 160 mg/kg/day. Increases in skeletal abnormalities in rabbits after IM administration of 5 mg/kg/day (estimated exposure was approximately 6 times the human daily sublingual dose of 16 mg) in the absence of maternal toxicity or oral administration of 1 mg/kg/day or greater (estimated exposure was approximately equal to the human sublingual dose of 16 mg) were not statistically significant.
In rabbits, buprenorphine produced statistically significant pre-implantation losses at oral doses of 1 mg/kg/day or greater and post-implantation losses that were statistically significant at IV doses of 0.2 mg/kg/day or greater (estimated exposure approximately 0.3 times the human daily sublingual dose of 16 mg). No maternal toxicity was noted at doses causing post-implantation loss in this study.
Dystocia was noted in pregnant rats treated intramuscularly with buprenorphine from Gestation Day 14 through Lactation Day 21 at 5 mg/kg/day (approximately 3 times the human sublingual dose of 16 mg). Fertility, pre- and post-natal development studies with buprenorphine in rats indicated increases in neonatal mortality after oral doses of 0.8 mg/kg/day and up (approximately 0.5 times the human daily sublingual dose of 16 mg), after IM doses of 0.5 mg/kg/day and up (approximately 0.3 times the human sublingual dose of 16 mg), and after SC doses of 0.1 mg/kg/day and up (approximately 0.06 times the human sublingual dose of 16 mg).
An apparent lack of milk production during these studies likely contributed to the decreased pup viability and lactation indices.
Delays in the occurrence of righting reflex and startle response were noted in rat pups at an oral dose of 80 mg/kg/day (approximately 50 times the human sublingual dose of 16 mg).
8.2 Lactation
Risk Summary
Based on two studies in 13 lactating women maintained on buprenorphine treatment, buprenorphine and its metabolite norbuprenorphine were present in low levels in human milk and infant urine.
Available data have not shown adverse reactions in breastfed infants. There are no data on the combination product buprenorphine/naloxone in breastfeeding, however oral absorption of naloxone is limited. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for SUBOXONE sublingual film and any potential adverse effects on the breastfed child from the drug or from the underlying maternal condition.
Clinical Considerations
Advise breastfeeding women taking buprenorphine products to monitor the infant for increased drowsiness and breathing difficulties.
Data
Data were consistent from two studies (N = 13) of breastfeeding infants whose mothers were maintained on sublingual doses of buprenorphine ranging from 2.4 to 24 mg/day, showing that the infants were exposed to less than 1% of the maternal daily dose.
In a study of six lactating women who were taking a median sublingual buprenorphine dose of 0.29 mg/kg/day 5 to 8 days after delivery, breast milk provided a median infant dose of 0.42 mcg/kg/day of buprenorphine and
0.33 mcg/kg/day of norbuprenorphine, equal to 0.2% and 0.12%, respectively, of the maternal weight-adjusted dose (relative dose/kg (%) of norbuprenorphine was calculated from the assumption that buprenorphine and norbuprenorphine are equipotent).
Data from a study of seven lactating women who were taking a median sublingual buprenorphine dose of 7 mg/day an average of 1.12 months after delivery indicated that the mean milk concentrations (C avg) of
buprenorphine and norbuprenorphine were 3.65 mcg/L and 1.94 mcg/L respectively. Based on the study data, and assuming milk consumption of 150 mL/kg/day, an exclusively breastfed infant would receive an estimated mean absolute infant dose (AID) of 0.55 mcg/kg/day of buprenorphine and 0.29 mcg/kg/day of norbuprenorphine, or a mean relative infant dose (RID) of 0.38% and 0.18%, respectively, of the maternal weight-adjusted dose.
8.4 Pediatric Use
The safety and effectiveness of SUBOXONE sublingual film have not been established in pediatric patients. This product is not appropriate for the treatment of neonatal abstinence syndrome in neonates, because it contains naloxone, an opioid antagonist.
8.5 Geriatric Use
Clinical studies of SUBOXONE sublingual film, SUBOXONE sublingual tablets, or SUBUTEX sublingual tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they responded differently than younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. Due to possible decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy in geriatric patients, the decision to prescribe SUBOXONE sublingual film should be made cautiously in individuals 65 years of age or older and these patients should be monitored for signs and symptoms of toxicity or overdose.
8.6 Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of buprenorphine and naloxone has been evaluated in a pharmacokinetic study. Both drugs are extensively metabolized in the liver. While no clinically significant changes have been observed in subjects with mild hepatic impairment; the plasma levels have been shown to be higher and half-life values have been shown to be longer for both buprenorphine and naloxone in subjects with moderate and severe hepatic impairment. The magnitude of the effects on naloxone are greater than that on buprenorphine in both moderately and severely impaired subjects. The difference in magnitude of the effects on naloxone and buprenorphine are greater in subjects with severe hepatic impairment than in subjects with moderate hepatic impairment, and therefore the clinical impact of these effects is likely to be greater in patients with severe hepatic impairment than in patients with moderate hepatic impairment.
Buprenorphine/naloxone products should be avoided in patients with severe hepatic impairment and may not be appropriate for patients with moderate hepatic impairment [see Warnings and Precautions ( 5.12), Clinical Pharmacology ( 12.3)].
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
SUBOXONE sublingual film contains buprenorphine, a Schedule III controlled substance under the Controlled Substances Act.
9.2 Abuse
Buprenorphine, like morphine and other opioids, has the potential for being abused and is subject to criminal diversion. This should be considered when prescribing or dispensing buprenorphine in situations when the clinician is concerned about an increased risk of misuse, abuse, or diversion. Healthcare professionals should contact their state professional licensing board or state controlled substances authority for information on how to prevent and detect abuse or diversion of this product.
Patients who continue to misuse, abuse, or divert buprenorphine products or other opioids should be provided with or referred for more intensive and structured treatment.
Abuse of buprenorphine poses a risk of overdose and death. This risk is increased with the abuse of buprenorphine and alcohol and other substances, especially benzodiazepines.
The healthcare provider may be able to more easily detect misuse or diversion by maintaining records of medication prescribed including date, dose, quantity, frequency of refills, and renewal requests of medication prescribed.
Proper assessment of the patient, proper prescribing practices, periodic re-evaluation of therapy, and proper handling and storage of the medication are appropriate measures that help to limit abuse of opioid drugs.
9.3 Dependence
Buprenorphine is a partial agonist at the mu-opioid receptor and chronic administration produces physical dependence of the opioid type, characterized by moderate withdrawal signs and symptoms upon abrupt discontinuation or rapid taper. The withdrawal syndrome is typically milder than seen with full agonists and may be delayed in onset [see Warnings and Precautions ( 5.7)] .
Neonatal opioid withdrawal syndrome (NOWS) is an expected and treatable outcome of prolonged use of opioids during pregnancy [see Warnings and Precautions ( 5.5)] .
-
10 OVERDOSAGE
Clinical Presentation
The manifestations of acute buprenorphine overdose include pinpoint pupils, sedation, hypotension, hypoglycemia, respiratory depression, and death.
Treatment of Overdose
In the event of overdose, the respiratory and cardiac status of the patient should be monitored carefully. When respiratory or cardiac functions are depressed, primary attention should be given to the re-establishment of adequate respiratory exchange through provision of a patent airway and institution of assisted or controlled ventilation. Oxygen, IV fluids, vasopressors, and other supportive measures should be employed as indicated.
In the case of overdose, the primary management should be the re-establishment of adequate ventilation with mechanical assistance of respiration, if required. Naloxone may be of value for the management of buprenorphine overdose. Higher than normal doses and repeated administration may be necessary. The long duration of action of SUBOXONE sublingual film should be taken into consideration when determining the length of treatment and medical surveillance needed to reverse the effects of an overdose. Insufficient duration of monitoring may put patients at risk.
-
11 DESCRIPTION
SUBOXONE® (buprenorphine and naloxone) sublingual film is an orange film, imprinted with a logo identifying the product and strength in white ink. It contains buprenorphine HCl, a mu-opioid receptor partial agonist, and a kappa- opioid receptor antagonist, and naloxone HCl dihydrate, an opioid antagonist, at a ratio of 4:1 (ratio of free bases). It is intended for sublingual or buccal administration and is available in four dosage strengths, 2 mg buprenorphine with 0.5 mg naloxone, 4 mg buprenorphine with 1 mg naloxone, 8 mg buprenorphine with 2 mg naloxone and 12 mg buprenorphine with 3 mg naloxone. Each film also contains polyethylene oxide, hydroxypropyl methylcellulose, maltitol, acesulfame potassium, lime flavor, citric acid, sodium citrate, FD&C yellow #6, and white ink.
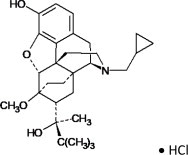
Chemically, buprenorphine HCl is (2S)-2-[17-Cyclopropylmethyl-4,5α-epoxy-3-hydroxy-6-methoxy-6α,14- ethano-14α-morphinan-7α-yl]-3,3-dimethylbutan-2-ol hydrochloride. It has the following chemical structure:
Buprenorphine HCl has the molecular formula C 29H 41NO 4• HCl and the molecular weight is 504.10. It is a white or off-white crystalline powder, sparingly soluble in water, freely soluble in methanol, soluble in alcohol, and practically insoluble in cyclohexane.
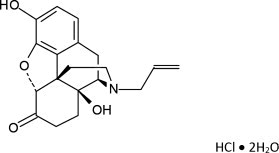
Chemically, naloxone HCl dihydrate is 17-Allyl-4,5 α -epoxy-3, 14-dihydroxymorphinan-6-one hydrochloride dihydrate. It has the following chemical structure:
Naloxone hydrochloride dihydrate has the molecular formula C 19H 21NO 4• HCl • 2H 2O and the molecular weight is 399.87. It is a white to slightly off-white powder and is freely soluble in water, soluble in alcohol, and practically insoluble in toluene and ether.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
SUBOXONE sublingual film contains buprenorphine and naloxone. Buprenorphine is a partial agonist at the mu-opioid receptor and an antagonist at the kappa-opioid receptor. Naloxone is a potent antagonist at mu opioid receptors and produces opioid withdrawal signs and symptoms in individuals physically dependent on full opioid agonists when administered parenterally.
12.2 Pharmacodynamics
Subjective Effects
Comparisons of buprenorphine to full opioid agonists such as methadone and hydromorphone suggest that sublingual buprenorphine produces typical opioid agonist effects which are limited by a ceiling effect.
In opioid-experienced subjects who were not physically dependent, acute sublingual doses of buprenorphine/naloxone tablets produced opioid agonist effects which reached a maximum between doses of 8 mg/2 mg and 16 mg/4 mg buprenorphine/naloxone.
Opioid agonist ceiling-effects were also observed in a double-blind, parallel group, dose-ranging comparison of single doses of buprenorphine sublingual solution (1, 2, 4, 8, 16, or 32 mg), placebo and a full agonist control at various doses. The treatments were given in ascending dose order at intervals of at least one week to 16 opioid-experienced subjects who were not physically dependent. Both active drugs produced typical opioid agonist effects. For all measures for which the drugs produced an effect, buprenorphine produced a dose- related response. However, in each case, there was a dose that produced no further effect. In contrast, the highest dose of the full agonist control always produced the greatest effects. Agonist objective rating scores remained elevated for the higher doses of buprenorphine (8 mg to 32 mg) longer than for the lower doses and did not return to baseline until 48 hours after drug administration. The onset of effects appeared more rapidly with buprenorphine than with the full agonist control, with most doses nearing peak effect after 100 minutes for buprenorphine compared to 150 minutes for the full agonist control.
Physiologic Effects
Buprenorphine in IV (2, 4, 8, 12 and 16 mg) and sublingual (12 mg) doses has been administered to opioid- experienced subjects who were not physically dependent to examine cardiovascular, respiratory, and subjective effects at doses comparable to those used for treatment of opioid dependence. Compared to placebo, there were no statistically significant differences among any of the treatment conditions for blood pressure, heart rate, respiratory rate, O 2saturation, or skin temperature across time. Systolic BP was higher in the 8 mg group than placebo (3 hour AUC values). Minimum and maximum effects were similar across all treatments. Subjects remained responsive to low voice and responded to computer prompts. Some subjects showed irritability, but no other changes were observed. The respiratory effects of sublingual buprenorphine were compared with the effects of methadone in a double- blind, parallel group, dose ranging comparison of single doses of buprenorphine sublingual solution (1, 2, 4, 8, 16, or 32 mg) and oral methadone (15, 30, 45, or 60 mg) in non-dependent, opioid-experienced volunteers. In this study, hypoventilation not requiring medical intervention was reported more frequently after buprenorphine doses of 4 mg and higher than after methadone. Both drugs decreased O 2saturation to the same degree.
Effect of Naloxone
Physiologic and subjective effects following acute sublingual administration of buprenorphine tablets and buprenorphine/naloxone tablets were similar at equivalent dose levels of buprenorphine. Naloxone had no clinically significant effect when administered by the sublingual route, although blood levels of the drug were measurable. Buprenorphine/naloxone, when administered sublingually to an opioid-dependent cohort, was recognized as an opioid agonist, whereas when administered intramuscularly, combinations of buprenorphine with naloxone produced opioid antagonist actions similar to naloxone. This finding suggests that the naloxone in buprenorphine/naloxone tablets may deter injection of buprenorphine/naloxone tablets by persons with active substantial heroin or other full mu-opioid dependence. However, clinicians should be aware that some opioid-dependent persons, particularly those with a low level of full mu-opioid physical dependence or those whose opioid physical dependence is predominantly to buprenorphine, abuse buprenorphine/naloxone combinations by the intravenous or intranasal route. In methadone-maintained patients and heroin- dependent subjects, IV administration of buprenorphine/naloxone combinations precipitated opioid withdrawal signs and symptoms and was perceived as unpleasant and dysphoric. In morphine-stabilized subjects, intravenously administered combinations of buprenorphine with naloxone produced opioid antagonist and withdrawal signs and symptoms that were ratio-dependent; the most intense withdrawal signs and symptoms were produced by 2:1 and 4:1 ratios, less intense by an 8:1 ratio.
Effects on the Endocrine System
Opioids inhibit the secretion of adrenocorticotropic hormone (ACTH), cortisol, and luteinizing hormone (LH) in humans [see Adverse Reactions ( 6.2)] . They also stimulate prolactin, growth hormone (GH) secretion, and pancreatic secretion of insulin and glucagon.
Chronic use of opioids may influence the hypothalamic-pituitary-gonadal axis, leading to androgen deficiency that may manifest as low libido, impotence, erectile dysfunction, amenorrhea, or infertility. The causal role of opioids in the clinical syndrome of hypogonadism is unknown because the various medical, physical, lifestyle, and psychological stressors that may influence gonadal hormone levels have not been adequately controlled for in studies conducted to date. Patients presenting with symptoms of androgen deficiency should undergo laboratory evaluation.
12.3 Pharmacokinetics
Absorption
In several pharmacokinetic studies following the administration of different dosages, a dose of one or two of the 2 mg/0.5 mg SUBOXONE sublingual films administered sublingually or buccally showed comparable relative bioavailability to the same total dose of SUBOXONE sublingual tablets. In contrast, one 8 mg/2 mg and one 12 mg/3 mg SUBOXONE sublingual films administered sublingually or buccally showed higher relative bioavailability for both buprenorphine and naloxone compared to the same total dose of SUBOXONE sublingual tablets. A combination of one 8 mg/2 mg and two 2 mg/0.5 mg SUBOXONE sublingual films (total dose of 12 mg/ 3 mg) administered sublingually showed comparable relative bioavailability to the same total dose of SUBOXONE sublingual tablets, while buccally administered SUBOXONE sublingual films showed higher relative bioavailability. Table 5, below, illustrates the relative increase in exposure to buprenorphine and naloxone associated with SUBOXONE sublingual films compared to SUBOXONE sublingual tablets, and shows the effect of route of administration [ see Dosage and Administration ( 2.9, 2.10) ].
Across relevant pharmacokinetic studies, the pharmacokinetic parameters and exposures derived from the buccal and sublingual administrations of SUBOXONE sublingual film were comparable to one another.
Table 5. Changes in Pharmacokinetic Parameters for SUBOXONE Sublingual Film Administered Sublingually or Buccally in Comparison to SUBOXONE Sublingual Tablet Note: 1. the 16 mg/4 mg strength film is not marketed; it is compositionally proportional to the 8 mg/2 mg strength film and has the same size of 2 x 8 mg/2 mg film. 2. – represents no change when the 90% confidence intervals for the geometric mean ratios of the Cmax and AUC0-last values are within the 80% to 125% limit. 3. There are no data for the 4 mg/1 mg strength film; it is compositionally proportional to 2 mg/0.5 mg strength film and has the same size of 2 x 2 mg/0.5 mg film strength.
Dosage PK
ParameterIncrease in Buprenorphine PK
ParameterIncrease in Naloxone Film Sublingual Compared to Tablet Sublingual Film Buccal Compared to Tablet Sublingual Film Buccal Compared to Film Sublingual Film Sublingual Compared to Tablet Sublingual Film Buccal Compared to Tablet Sublingual Film Buccal Compared to Film Sublingual 1 x 2 mg/0.5 mg C max 22% 25% - C max - - - AUC 0-last - 19% - AUC 0-last - - - 2 x 2 mg/0.5 mg C max - 21% 21% C max - 17% 21% AUC 0-last - 23% 16% AUC 0-last - 22% 24% 1 x 8 mg/2 mg C max 28% 34% - C max 41% 54% - AUC 0-last 20% 25% - AUC 0-last 30% 43% - 1 x 12 mg/3 mg C max 37% 47% - C max 57% 72% 9% AUC 0-last 21% 29% - AUC 0-last 45% 57% - 1 x 8 mg/2 mg plus
2 x 2 mg/0.5 mgC max - 27% 13% C max 17% 38% 19% AUC 0-last - 23% - AUC 0-last - 30% 19% 1 x 16 mg/4 mg film C max 34% 29% 7% C max 44% 46% 9% AUC 0-last 32% - - AUC 0-last 49% 36% 3% Distribution
Buprenorphine is approximately 96% protein bound, primarily to alpha and beta globulin. Naloxone is approximately 45% protein bound, primarily to albumin.
Elimination
Buprenorphine is metabolized and eliminated in urine and feces. Naloxone undergoes metabolism as well. When SUBOXONE sublingual film is administered sublingually or buccally, buprenorphine has a mean elimination half-life ranging from 24 to 42 hours and naloxone has a mean elimination half-life ranging from 2 to 12 hours.
Metabolism
Buprenorphine undergoes both N-dealkylation to norbuprenorphine and glucuronidation. The N-dealkylation pathway is mediated primarily by the CYP3A4. Norbuprenorphine, the major metabolite, can further undergo
glucuronidation. Norbuprenorphine has been found to bind opioid receptors in vitro; however, it has not been studied clinically for opioid-like activity. Naloxone undergoes direct glucuronidation to naloxone-3-glucuronide as well as N-dealkylation, and reduction of the 6-oxo group.
Excretion
A mass balance study of buprenorphine showed complete recovery of radiolabel in urine (30%) and feces (69%) collected up to 11 days after dosing. Almost all of the dose was accounted for in terms of buprenorphine, norbuprenorphine, and two unidentified buprenorphine metabolites. In urine, most of buprenorphine and norbuprenorphine was conjugated (buprenorphine, 1% free and 9.4% conjugated; norbuprenorphine, 2.7% free and 11% conjugated). In feces, almost all of the buprenorphine and norbuprenorphine were free (buprenorphine, 33% free and 5% conjugated; norbuprenorphine, 21% free and 2% conjugated). Based on all studies performed with sublingually and buccally administered SUBOXONE sublingual film, buprenorphine has a mean elimination half-life from plasma ranging from 24 to 42 hours and naloxone has a mean elimination half-life from plasma ranging from 2 to 12 hours.
Drug Interactions Studies
CYP3A4 Inhibitors and Inducers
Buprenorphine has been found to be a CYP2D6 and CYP3A4 inhibitor and its major metabolite, norbuprenorphine, has been found to be a moderate CYP2D6 inhibitor in in vitrostudies employing human liver microsomes. However, the relatively low plasma concentrations of buprenorphine and norbuprenorphine resulting from therapeutic doses are not expected to raise significant drug-drug interaction concerns [see Drug Interactions ( 7)] .
Hepatic Impairment
In a pharmacokinetic study, the disposition of buprenorphine and naloxone were determined after administering a 2.0/0.5 mg SUBOXONE sublingual tablet in subjects with varied degrees of hepatic impairment as indicated by Child-Pugh criteria. The disposition of buprenorphine and naloxone in patients with hepatic impairment were compared to disposition in subjects with normal hepatic function.
In subjects with mild hepatic impairment, the changes in mean C max, AUC 0-last, and half-life values of both buprenorphine and naloxone were not clinically significant. No dosing adjustment is needed in patients with mild hepatic impairment.
For subjects with moderate and severe hepatic impairment, mean C max, AUC 0-last, and half-life values of both buprenorphine and naloxone were increased; the effects on naloxone are greater than that on buprenorphine ( Table 6).
Table 6. Changes in Pharmacokinetic Parameters in Subjects With Moderate and Severe Hepatic Impairment Hepatic Impairment PK Parameters Increase in buprenorphine compared to healthy subjects Increase in naloxone compared to healthy subjects Moderate C max 8% 170% AUC 0-last 64% 218% Half-life 35% 165% Severe C max 72% 1030% AUC 0-last 181% 1302% Half-life 57% 122% The difference in magnitude of the effects on naloxone and buprenorphine are greater in subjects with severe hepatic impairment than subjects with moderate hepatic impairment [see Warnings and Precautions ( 5.12), Use in Specific Populations ( 8.6)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
Carcinogenicity data on SUBOXONE sublingual film are not available.
A carcinogenicity study of buprenorphine/naloxone (4:1 ratio of the free bases) was performed in Alderley Park rats. Buprenorphine/naloxone was administered in the diet at doses of approximately 7, 31, and
123 mg/kg/day for 104 weeks (estimated exposure was approximately 4, 18, and 44 times the recommended human sublingual dose of 16 mg/4 mg buprenorphine/naloxone based on buprenorphine AUC comparisons). A statistically significant increase in Leydig cell adenomas was observed in all dose groups. No other drug-related tumors were noted.
Carcinogenicity studies of buprenorphine were conducted in Sprague-Dawley rats and CD-1 mice. Buprenorphine was administered in the diet to rats at doses of 0.6, 5.5, and 56 mg/kg/day (estimated exposure was approximately 0.4, 3, and 35 times the recommended human daily sublingual dose of 16 mg on a mg/m 2basis) for 27 months. As in the buprenorphine/naloxone carcinogenicity study in rats, statistically significant dose-related increases in Leydig cell tumors occurred. In an 86 week study in CD-1 mice, buprenorphine was not carcinogenic at dietary doses up to 100 mg/kg/day (estimated exposure was approximately 30 times the recommended human daily sublingual dose of 16 mg on a mg/m 2basis).
Mutagenicity
The 4:1 combination of buprenorphine and naloxone was not mutagenic in a bacterial mutation assay (Ames test) using four strains of S. typhimuriumand two strains of E. coli.The combination was not clastogenic in an in vitrocytogenetic assay in human lymphocytes or in an IV micronucleus test in the rat.
Buprenorphine was studied in a series of tests utilizing gene, chromosome, and DNA interactions in both prokaryotic and eukaryotic systems. Results were negative in yeast ( S. cerevisiae)for recombinant, gene convertant, or forward mutations; negative in Bacillus subtilis“rec” assay, negative for clastogenicity in CHO cells, Chinese hamster bone marrow and spermatogonia cells, and negative in the mouse lymphoma L5178Y assay.
Results were equivocal in the Ames test: negative in studies in two laboratories, but positive for frame shift mutation at a high dose (5 mg/plate) in a third study. Results were positive in the Green-Tweets ( E. coli) survival test, positive in a DNA synthesis inhibition (DSI) test with testicular tissue from mice, for both in vivoand in vitroincorporation of [ 3H]thymidine, and positive in unscheduled DNA synthesis (UDS) test using testicular cells from mice.
Impairment of Fertility
Dietary administration of buprenorphine in the rat at dose levels of 500 ppm or greater (equivalent to approximately 47 mg/kg/day or greater; estimated exposure approximately 28 times the recommended human daily sublingual dose of 16 mg on a mg/m 2basis) produced a reduction in fertility demonstrated by reduced female conception rates. A dietary dose of 100 ppm (equivalent to approximately 10 mg/kg/day; estimated exposure approximately 6 times the recommended human daily sublingual dose of 16 mg on a mg/m 2basis) had no adverse effect on fertility.
-
16 HOW SUPPLIED / STORAGE AND HANDLING
SUBOXONE sublingual film is supplied as an orange rectangular film with a white printed logo in child-resistant polyester/foil laminated pouches:
- NDC 12496-1202-3 (buprenorphine 2 mg/naloxone 0.5 mg/film; content expressed in terms of free base, equivalent to 2.16 mg buprenorphine hydrochloride USP and 0.61 mg naloxone hydrochloride dihydrate USP) - 30 films per carton
- NDC 12496-1204-3 (buprenorphine 4 mg/naloxone 1 mg/film; content expressed in terms of free base, equivalent to 4.32 mg buprenorphine hydrochloride USP and 1.22 mg naloxone hydrochloride dihydrate USP) - 30 films per carton
- NDC 12496-1208-3 (buprenorphine 8 mg/naloxone 2 mg/film; content expressed in terms of free base, equivalent to 8.64 mg buprenorphine hydrochloride USP and 2.44 mg naloxone hydrochloride dihydrate USP) - 30 films per carton
- NDC 12496-1212-3 (buprenorphine 12 mg/naloxone 3 mg/film; content expressed in terms of free base, equivalent to 12.96 mg buprenorphine hydrochloride USP and 3.66 mg naloxone hydrochloride dihydrate USP) - 30 films per carton
Store at 25°C (77°F), excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature].
Store SUBOXONE sublingual film securely and dispose of properly [see Patient Counseling Information ( 17)] .
-
17 PATIENT COUNSELING INFORMATION
Advise patients to read the FDA-approved patient labeling ( Medication Guide).
Storage and Disposal
Because of the risks associated with accidental ingestion, misuse, and abuse, advise patients to store SUBOXONE sublingual film securely, out of sight and reach of children, and in a location not accessible by others, including visitors to the home [see Warnings and Precautions ( 5.1, 5.4), Drug Abuse and Dependence ( 9.2)] . Inform patients that leaving SUBOXONE sublingual film unsecured can pose a deadly risk to others in the home.
Advise patients and caregivers that when medicines are no longer needed, they should be disposed of promptly. Expired, unwanted, or unused SUBOXONE sublingual film should be disposed of by removing the SUBOXONE sublingual film from the foil packaging, and flushing the unused medication down the toilet (if a drug take-back option is not readily available). Inform patients that they can visit www.fda.gov/drugdisposal for a complete list of medicines recommended for disposal by flushing, as well as additional information on disposal of unused medicines.
Safe Use
Before initiating treatment with SUBOXONE sublingual film, explain the points listed below to caregivers and patients. Instruct patients to read the Medication Guide each time SUBOXONE sublingual film is dispensed because new information may be available.
- SUBOXONE sublingual film must be administered whole. Advise patients not to cut, chew, or swallow SUBOXONE sublingual film.
- Inform patients and caregivers that potentially fatal additive effects may occur if SUBOXONE sublingual film is used with benzodiazepines or other CNS depressants, including alcohol. Counsel patients that such medications should not be used concomitantly unless supervised by a health care provider [see Warnings and Precautions ( 5.2, 5.3), Drug Interactions ( 7)].
- Educate patients and caregivers on how to recognize respiratory depression and emphasize the importance of calling 911 or getting emergency medical help right away in the event of a known or suspected overdose [see Warnings and Precautions ( 5.2)].
-
Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose
Because patients being treated for opioid use disorder are at risk for relapse, discuss the importance of having access to naloxone with the patient and caregiver. Also discuss the importance of having access to naloxone if there are household members (including children) or other close contacts at risk for accidental ingestion or opioid overdose.
Inform patients and caregivers of the options for obtaining naloxone as permitted by individual state naloxone dispensing and prescribing requirements or guidelines (e.g., by prescription, directly from a pharmacist, or as part of a community-based program). Educate patients and caregivers on how to recognize the signs and symptoms of an opioid overdose.
Explain to patients and caregivers that naloxone's effects are temporary, and that they must call 911 or get emergency medical help right away in all cases of known or suspected opioid overdose, even if naloxone is administered. Repeat administration may be necessary, particularly for overdose involving SUBOXONE sublingual film, because naloxone is often not effective at the doses available for patient access [see Dosage and Administration ( 2.2), Warnings and Precautions ( 5.2), Overdosage ( 10)] .
If naloxone is prescribed, also advise patients and caregivers:- How to treat with naloxone in the event of an opioid overdose
- To tell family and friends about their naloxone and to keep it in a place where family and friends can easily access it in an emergency
- To read the Patient Information (or other educational material) that will come with their naloxone. Emphasize the importance of doing this before an opioid emergency happens, so the patient and caregiver will know what to do.
- Advise patients that SUBOXONE sublingual film contains an opioid that can be a target for people who abuse prescription medications or street drugs. Caution patients to keep their films in a safe place, and to protect them from theft.
- Instruct patients to keep SUBOXONE sublingual film in a secure place, out of the sight and reach of children. Accidental or deliberate ingestion by a child may cause respiratory depression that can result in death. Advise patients to seek medical attention immediately if a child is exposed to SUBOXONE sublingual film.
- Inform patients that opioids could cause a rare but potentially life-threatening condition resulting from concomitant administration of serotonergic drugs. Warn patients of the symptoms of serotonin syndrome and to seek medical attention right away if symptoms develop. Instruct patients to inform their healthcare providers if they are taking, or plan to take serotonergic medications [see Drug Interactions ( 7)] .
- Inform patients that opioids could cause adrenal insufficiency, a potentially life- threatening condition. Adrenal insufficiency may present with non-specific symptoms and signs such as nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. Advise patients to seek medical attention if they experience a constellation of these symptoms [see Warnings and Precautions ( 5.6)] .
- Advise patients to never give SUBOXONE sublingual film to anyone else, even if he or she has the same signs and symptoms. It may cause harm or death.
- Advise patients that selling or giving away this medication is against the law.
- Advise patients that, after SUBOXONE has completely dissolved in the oral mucosa, to take a sip of water, swish it gently around their teeth and gums, and swallow. Advise patients to wait for at least one hour after taking SUBOXONE before brushing teeth [see Warnings and Precautions ( 5.13)] .
- Refer patients to dental care services and encourage them to have regular dental checkups while taking SUBOXONE. Instruct patients to inform their dentist that they have started therapy on SUBOXONE [see Warnings and Precautions ( 5.13)] .
- Caution patients that SUBOXONE sublingual film may impair the mental or physical abilities required for the performance of potentially dangerous tasks such as driving or operating machinery. Caution should be taken especially during drug induction and dose adjustment and until individuals are reasonably certain that buprenorphine therapy does not adversely affect their ability to engage in such activities [see Warnings and Precautions ( 5.15)].
- Advise patients not to change the dosage of SUBOXONE sublingual film without consulting their healthcare provider.
- Advise patients to take SUBOXONE sublingual film once a day.
- Advise patients that if they miss a dose of SUBOXONE sublingual film they should take it as soon as they remember. If it is almost time for the next dose, they should skip the missed dose and take the next dose at the regular time.
- Inform patients that SUBOXONE sublingual film can cause drug dependence and that withdrawal signs and symptoms may occur when the medication is discontinued.
- Advise patients seeking to discontinue treatment with buprenorphine for opioid dependence to work closely with their healthcare provider on a tapering schedule and inform them of the potential to relapse to illicit drug use associated with discontinuation of opioid agonist/partial agonist medication-assisted treatment.
- Advise patients that, like other opioids, SUBOXONE sublingual film may produce orthostatic hypotension in ambulatory individuals [see Warnings and Precautions ( 5.16)].
- Advise patients to inform their healthcare provider if any other prescription medications, over-the-counter medications, or herbal preparations are prescribed or currently being used [see Drug Interactions ( 7)].
- Advise women that if they are pregnant while being treated with SUBOXONE sublingual film, the baby may have signs of withdrawal at birth and that withdrawal is treatable [see Warnings and Precautions ( 5.5), Use in Specific Populations ( 8.1)].
- Advise women who are breastfeeding to monitor the infant for drowsiness and difficulty breathing [see Use in Specific Populations ( 8.2)].
- Inform patients that chronic use of opioids may cause reduced fertility. It is not known whether these effects on fertility are reversible [see Use in Specific Populations ( 8.3)].
- Advise patients to inform their family members that, in the event of emergency, the treating healthcare provider or emergency room staff should be informed that the patient is physically dependent on an opioid and that the patient is being treated with SUBOXONE sublingual film.
© 2023, Indivior UK Limited. All Rights Reserved.
SUBOXONE ®and SUBUTEX ®are registered trademarks of Indivior UK Limited.
Manufactured for Indivior Inc.
North Chesterfield, VA 23235 by:
Aquestive Therapeutics,
Warren, NJ 07059
Distributed by:
Indivior Inc.
North Chesterfield, VA 23235
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: 06/2022
MEDICATION GUIDE
SUBOXONE (Sub-OX-own) (buprenorphine and naloxone) Sublingual Film, CIIIIMPORTANT:Keep SUBOXONE sublingual film in a secure place away from children. Accidental use by a child is a medical emergency and can result in death. If a child accidentally takes SUBOXONE sublingual film, get emergency help or call 911 right away. Tell your healthcare provider if you are living in a household where there are small children. What is the most important information I should know about SUBOXONE sublingual film?
- SUBOXONE contains a medicine called buprenorphine. Buprenorphine is an opioid that can cause serious and life-threatening breathing problems, especially if you take or use certain other medicines or drugs.
- Talk to your healthcare provider about naloxone. Naloxone is a medicine that is available to patients for the emergency treatment of an opioid overdose, including accidental use of SUBOXONE sublingual film by a child. If naloxone is given, you must call 911 or get emergency medical help right away to treat an overdose or accidental use of an opioid.
- SUBOXONE may cause serious and life-threatening breathing problems. Get emergency help right away if you:
- feel faint
- feel dizzy
- are confused
- feel sleepy or uncoordinated
- have blurred vision
- have slurred speech
- are breathing slower than normal
- cannot think well or clearly
- Do not take SUBOXONE with certain medicines. Taking SUBOXONE with other opioid medicines, benzodiazepines, alcohol, or other central nervous system depressants (including street drugs) can cause severe drowsiness, decreased awareness, breathing problems, coma, and death.
- Do not inject (“shoot-up”) SUBOXONE. Injecting SUBOXONE may cause life-threatening infections and other serious health problems, Injecting SUBOXONE may cause sudden serious withdrawal symptoms such as pain, cramps, vomiting, diarrhea, anxiety, sleep problems, and cravings.
- Do not switch from SUBOXONE sublingual film to other medicines that contain buprenorphinewithout talking with your healthcare provider. The amount of buprenorphine in a dose of SUBOXONE sublingual film is not the same as in other medicines that contain buprenorphine. Your healthcare provider will prescribe a starting dose of SUBOXONE sublingual film that may be different than other buprenorphine containing medicines you may have been taking.
- Do not stop taking SUBOXONE suddenly. You could become sick and have withdrawal symptoms because your body has become used to the medicine (physical dependence). Physical dependence is not the same as drug addiction.
- In an emergency, have family members tell emergency department staff that you are physically dependent on an opioid and are being treated with SUBOXONE sublingual film.
- Never give anyone else your SUBOXONE sublingual film. They could die from taking it. Selling or giving away SUBOXONE sublingual film is against the law.
- Store SUBOXONE sublingual film securely, out of sight and reach of children, and in a location not accessible by others, including visitors to the home.
What is SUBOXONE sublingual film?
- SUBOXONE sublingual film is a prescription medicine used to treat opioid addiction in adults and is part of a complete treatment program that also includes counseling and behavioral therapy.
Who should not take SUBOXONE sublingual film?
Do not take SUBOXONE sublingual filmif you are allergic to buprenorphine or naloxone.Before taking SUBOXONE, tell your healthcare provider about all your medical conditions, including if you have:
- trouble breathing or lung problems
- a curve in your spine that affects your breathing
- Addison's disease
- an enlarged prostate (men)
- problems urinating
- liver, kidney, or gallbladder problems
- alcoholism
- a head injury or brain problem
- mental health problems
- adrenal gland or thyroid gland problems
- tooth problems, including a history of cavities
Tell your healthcare provider if you are:
- pregnant or plan to become pregnant.If you take SUBOXONE while pregnant, your baby may have symptoms of opioid withdrawal at birth that could be life-threatening if not recognized and treated. Talk to your healthcare provider if you are pregnant or plan to become pregnant.
-
Breastfeeding or plan to breastfeed.SUBOXONE can pass into your breast milk and harm your baby. Talk to your healthcare provider about the best way to feed your baby if you take SUBOXONE. Monitor your baby for increased drowsiness and breathing problems if you breastfeed during treatment with SUBOXONE.
Tell your healthcare provider about all the medicines you take, includingprescription and over-the-counter medicines, vitamins, or herbal supplements. How should I take SUBOXONE sublingual film?
Read the Instructions for Useat the end of this Medication Guide for detailed instructions on how to take SUBOXONE
- Take SUBOXONE sublingual film exactly as prescribed by your healthcare provider. Your healthcare provider may change your dose after seeing how it affects you. Do not change your dose unless your healthcare provider tells you to change it.
- Do not take SUBOXONE sublingual film more often than prescribed by your healthcare provider.
- SUBOXONE is not for occasional or “as needed” use.
- When you are beginning treatment, take SUBOXONE sublingual film only under the tongue (sublingual administration).
- After SUBOXONE is completely dissolved, rinse your mouth with water and swallow. Wait for at least one hour before brushing teeth.
- Report any problems with your teeth immediately to your healthcare provider and schedule an appointment with a dentist. Tell your dentist that you have started taking SUBOXONE.
- After a few days, you can choose whether you will take SUBOXONE sublingual film on the inside of your cheek (buccal administration) or under the tongue (sublingual administration).
- Take the entire SUBOXONE sublingual film. Do not cut, tear, chew, or swallow SUBOXONE sublingual film because the medicine will not work as well.
- If you miss a dose of SUBOXONE, take it as soon as you remember. If it is almost time for your next dose, skip the missed dose and take the next dose at your regular time. Do not take 2 doses at the same time unless your healthcare provider tells you to. If you are not sure about your dosing, call your healthcare provider.
- Dispose of expired, unwanted, or unused SUBOXONE by removing SUBOXONE from the foil packaging, and promptly flushing down the toilet (if a drug take-back option is not readily available). Visit www.fda.gov/drugdisposal for additional information on disposal of unused medicines.
- If you take too much SUBOXONE or overdose, call Poison Control or get emergency medical help right away.
What should I avoid while taking SUBOXONE sublingual film?
- Do not drive, operate heavy machinery, or perform any other dangerous activities until you know how SUBOXONE affects you. Buprenorphine can cause drowsiness and slow reaction times. SUBOXONE can make you sleepy, dizzy, or lightheaded.
- You should not drink alcoholor take prescription or over-the-counter medicines that contain alcohol while taking SUBOXONE sublingual film, because this can lead to loss of consciousness or even death.
What are the possible side effects of SUBOXONE sublingual film?
SUBOXONE sublingual film can cause serious side effects, including:
- Trouble breathing.Taking SUBOXONE with other opioid medicines, benzodiazepines, alcohol, or other central nervous system depressants can cause breathing problems that can lead to coma and death.
- Sleepiness, dizziness, and problems with coordination.
- Physical dependence or abuse.
-
Liver problems. Call your healthcare provider right away if you notice any of these symptoms:
- your skin or the white part of your eyes turns yellow (jaundice)
- dark or “tea-colored” urine
- light colored stools (bowel movements)
- loss of appetite
- pain, aching, or tenderness on the right side of your stomach area
- nausea
- Your healthcare provider should do blood tests to check your liver before you start taking and while you take SUBOXONE.
- Allergic reaction.You may have a rash, hives, swelling of your face, wheezing, low blood pressure, or loss of consciousness. Call your healthcare provider or get emergency help right away.
-
Opioid withdrawal.Call your healthcare provider right away if you get any of these symptoms:
- shaking
- sweating more than normal
- feeling hot or cold more than normal
- runny nose
- watery eyes
- goose bumps
- diarrhea
- vomiting
- muscle aches
- Decrease in blood pressure.You may feel dizzy if you get up too fast from sitting or lying down.
-
The most common side effects of SUBOXONE include:
- headache
- nausea
- vomiting
- constipation
- pain
- increased sweating
- decrease in sleep (insomnia)
- SUBOXONE sublingual film may affect fertility in males and females. Talk to your healthcare provider if this is a concern for you.
These are not all the possible side effects of SUBOXONE.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about the safe and effective use of SUBOXONE sublingual film.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not take SUBOXONE sublingual film for a condition for which it was not prescribed. Do not give SUBOXONE sublingual film to other people, even if they have the same symptoms you have. It may harm them and it is against the law.
You can ask your doctor or pharmacist for information that is written for healthcare professionals.Manufactured for Indivior Inc., North Chesterfield, VA 23235 by Aquestive Therapeutics, Warren, NJ 07059
SUBOXONE ®is a registered trademark of Indivior UK Limited.
For more information, go to www.SUBOXONE.comor call 1‐877‐782‐6966. -
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
SUBOXONE (Sub-OX-own) (buprenorphine and naloxone) Sublingual Film, CIII
This “Instructions for Use” contains information on how to correctly take SUBOXONE sublingual film.
Important Information You Need to Know Before Taking SUBOXONE sublingual film:
- Your healthcare provider should show you how to take SUBOXONE sublingual film the right way.
- Each SUBOXONE sublingual film comes in a sealed child‐resistant foil pouch. Do not open the foil pouch until you are ready to take it.
Preparing to take SUBOXONEsublingual film:
-
To open your SUBOXONE sublingual film foil pouch, fold along the dotted line (see Figure 1).
-
Tear down at slit or cut with scissors along the arrow (see Figure 2).
- Before taking SUBOXONE sublingual film, drink water to moisten your mouth. This helps the film dissolve more easily.
To take SUBOXONE sublingual film under your tongue (sublingual administration):
- Hold the film between two fingers by the outside edges.
-
Place the SUBOXONE sublingual film under your tongue, close to the base either to the left or right of the center (see Figure 3).
- If your healthcare provider tells you to take 2 films at a time, place the second film under your tongue on the opposite side. Avoid letting the films touch.
- Keep the films in place until they have completely dissolved.
- If your healthcare provider tells you to take a third film, place it under your tongue on either side after the first 2 films have dissolved.
To take SUBOXONE sublingual film on the inside of your cheek (buccal administration):
- Hold the film between two fingers by the outside edges.
-
Place one film on the inside of your right or left cheek (see Figure 4).
- If your healthcare provider tells you to take 2 films at a time, place the other film on the inside of the opposite cheek.
- Keep the films in place until they have completely dissolved.
- If your healthcare provider tells you to take a third film, place it on the inside of your right or left cheek after the first 2 films have dissolved.
- While SUBOXONE sublingual film is dissolving, do not chew or swallow the film because the medicine will not work as well.
- Talking while the film is dissolving can affect how well the medicine in SUBOXONE sublingual film is absorbed.
- After SUBOXONE is completely dissolved, rinse your mouth with water and swallow. Wait for at least one hour before brushing teeth.
- If you miss a dose of SUBOXONE sublingual film, take your medicine when you remember. If it is almost time for your next dose, skip the missed dose and take the next dose at your regular time. Do not take 2 doses at the same time unless your healthcare provider tells you to. If you are not sure about your dosing, call your healthcare provider.
- Do not stop taking SUBOXONE sublingual film suddenly. You could become sick and have withdrawal symptoms because your body has become used to the medicine. Physical dependence is not the same as drug addiction. Your healthcare provider can tell you more about the differences between physical dependence and drug addiction. To have fewer withdrawal symptoms, ask your healthcare provider how to stop using SUBOXONE sublingual film the right way.
If you take too much SUBOXONE sublingual film or overdose, call Poison Control or get emergency medical help right away.
Storing SUBOXONE Sublingual Film:
- Store SUBOXONE sublingual film at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep SUBOXONE sublingual film in a safe place, out of the sight and reach of children.
Disposing of SUBOXONE Sublingual Film:
- Dispose of unused SUBOXONE sublingual film as soon as you no longer need them.
- Dispose of expired, unwanted or unused SUBOXONE sublingual film by removing the SUBOXONE sublingual film from the foil packaging, and promptly flushing down the toilet (if a drug take-back option is not readily available). Do not flush the SUBOXONE sublingual film foil pouch down the toilet. Visit www.fda.gov/drugdisposalfor additional information on disposal of unused medicines.
If you need help with disposal of SUBOXONE sublingual film, call 1‐877‐782‐6966.
SUBOXONE ®is a registered trademark of Indivior UK Limited.
This “Instructions for Use” has been approved by the U.S. Food and Drug Administration.
Revised 06/2022 -
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 2 mg Carton Label
NDC 12496-1202-3
2 mg/0.5 mg CIII
30 pouches each containing
1 sublingual filmSuboxone®
(buprenorphine and naloxone) sublingual film2 mg/0.5 mg
Rx only
Children who accidentally take SUBOXONE will
need emergency medical care. Keep SUBOXONE
out of the reach of children.Do not cut, chew or
swallow sublingual film.suboxone.com
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 4 mg Carton Label
NDC 12496-1204-3
4 mg/1 mg CIII
30 pouches each containing
1 sublingual filmSuboxone®
(buprenorphine and naloxone) sublingual film4 mg/1 mg
Rx only
Children who accidentally take SUBOXONE will
need emergency medical care. Keep SUBOXONE
out of the reach of children.Do not cut, chew or
swallow sublingual film.suboxone.com
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 8 mg Carton Label
NDC 12496-1208-3
8 mg/2 mg CIII
30 pouches each containing
1 sublingual filmSuboxone®
(buprenorphine and naloxone) sublingual film8 mg/2 mg
Rx only
Children who accidentally take SUBOXONE will
need emergency medical care. Keep SUBOXONE
out of the reach of children.Do not cut, chew or
swallow sublingual film.suboxone.com
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel –12 mg Carton Label
NDC 12496-1212-3
12 mg/3 mg CIII
30 pouches each containing
1 sublingual filmSuboxone®
(buprenorphine and naloxone) sublingual film12 mg/3 mg
Rx only
Children who accidentally take SUBOXONE will
need emergency medical care. Keep SUBOXONE
out of the reach of children.Do not cut, chew or
swallow sublingual film.suboxone.com
-
INGREDIENTS AND APPEARANCE
SUBOXONE
buprenorphine hydrochloride, naloxone hydrochloride film, solubleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:12496-1202 Route of Administration SUBLINGUAL, BUCCAL DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUPRENORPHINE HYDROCHLORIDE (UNII: 56W8MW3EN1) (BUPRENORPHINE - UNII:40D3SCR4GZ) BUPRENORPHINE 2 mg NALOXONE HYDROCHLORIDE (UNII: F850569PQR) (NALOXONE - UNII:36B82AMQ7N) NALOXONE 0.5 mg Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) HYPROMELLOSES (UNII: 3NXW29V3WO) MALTITOL (UNII: D65DG142WK) ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE (UNII: 1Q73Q2JULR) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:12496-1202-3 30 in 1 CARTON 09/13/2010 1 NDC:12496-1202-1 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022410 09/13/2010 SUBOXONE
buprenorphine hydrochloride, naloxone hydrochloride film, solubleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:12496-1204 Route of Administration SUBLINGUAL, BUCCAL DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUPRENORPHINE HYDROCHLORIDE (UNII: 56W8MW3EN1) (BUPRENORPHINE - UNII:40D3SCR4GZ) BUPRENORPHINE 4 mg NALOXONE HYDROCHLORIDE (UNII: F850569PQR) (NALOXONE - UNII:36B82AMQ7N) NALOXONE 1 mg Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) HYPROMELLOSES (UNII: 3NXW29V3WO) MALTITOL (UNII: D65DG142WK) ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE (UNII: 1Q73Q2JULR) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:12496-1204-3 30 in 1 CARTON 08/24/2012 1 NDC:12496-1204-1 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022410 08/24/2012 SUBOXONE
buprenorphine hydrochloride, naloxone hydrochloride film, solubleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:12496-1208 Route of Administration SUBLINGUAL, BUCCAL DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUPRENORPHINE HYDROCHLORIDE (UNII: 56W8MW3EN1) (BUPRENORPHINE - UNII:40D3SCR4GZ) BUPRENORPHINE 8 mg NALOXONE HYDROCHLORIDE (UNII: F850569PQR) (NALOXONE - UNII:36B82AMQ7N) NALOXONE 2 mg Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) HYPROMELLOSES (UNII: 3NXW29V3WO) MALTITOL (UNII: D65DG142WK) ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE (UNII: 1Q73Q2JULR) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:12496-1208-3 30 in 1 CARTON 09/13/2010 1 NDC:12496-1208-1 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022410 09/13/2010 SUBOXONE
buprenorphine hydrochloride, naloxone hydrochloride film, solubleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:12496-1212 Route of Administration SUBLINGUAL, BUCCAL DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUPRENORPHINE HYDROCHLORIDE (UNII: 56W8MW3EN1) (BUPRENORPHINE - UNII:40D3SCR4GZ) BUPRENORPHINE 12 mg NALOXONE HYDROCHLORIDE (UNII: F850569PQR) (NALOXONE - UNII:36B82AMQ7N) NALOXONE 3 mg Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) HYPROMELLOSES (UNII: 3NXW29V3WO) MALTITOL (UNII: D65DG142WK) ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE (UNII: 1Q73Q2JULR) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:12496-1212-3 30 in 1 CARTON 08/24/2012 1 NDC:12496-1212-1 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022410 08/24/2012 Labeler - INDIVIOR INC. (797408549) Registrant - INDIVIOR INC. (797408549) Establishment Name Address ID/FEI Business Operations Alcami Carolinas Corporation 117877975 analysis(12496-1202, 12496-1204, 12496-1208, 12496-1212) Establishment Name Address ID/FEI Business Operations Alcami Carolinas Corporation 831351445 analysis(12496-1202, 12496-1204, 12496-1208, 12496-1212) Establishment Name Address ID/FEI Business Operations Aquestive Therapeutics 079269181 manufacture(12496-1202, 12496-1204, 12496-1208, 12496-1212) , analysis(12496-1202, 12496-1204, 12496-1208, 12496-1212) , pack(12496-1202, 12496-1204, 12496-1208, 12496-1212) Establishment Name Address ID/FEI Business Operations Butterworth Laboratories Ltd 225081538 analysis(12496-1202, 12496-1204, 12496-1208, 12496-1212) Establishment Name Address ID/FEI Business Operations INDIVIOR UK Limited 220689622 analysis(12496-1202, 12496-1204, 12496-1208, 12496-1212) , api manufacture(12496-1202, 12496-1204, 12496-1208, 12496-1212) Establishment Name Address ID/FEI Business Operations Infinity Labs IN, Inc 118120524 analysis(12496-1202, 12496-1204, 12496-1208, 12496-1212) Establishment Name Address ID/FEI Business Operations Macfarlan Smith Limited 214057671 api manufacture(12496-1202, 12496-1204, 12496-1208, 12496-1212) Establishment Name Address ID/FEI Business Operations PPD Development, L.P. 838082055 analysis(12496-1202, 12496-1204, 12496-1208, 12496-1212) Establishment Name Address ID/FEI Business Operations QUOTIENT SCIENCES (ALNWICK) LIMITED 228248590 analysis(12496-1202, 12496-1204, 12496-1208, 12496-1212) Establishment Name Address ID/FEI Business Operations SGS North America Inc. 049859261 analysis(12496-1202, 12496-1204, 12496-1208, 12496-1212) Establishment Name Address ID/FEI Business Operations Sharp Packaging Services, LLC 143696495 pack(12496-1202, 12496-1204, 12496-1208, 12496-1212) Establishment Name Address ID/FEI Business Operations Siegfried AG 482824026 api manufacture(12496-1202, 12496-1204, 12496-1208, 12496-1212) , analysis(12496-1202, 12496-1204, 12496-1208, 12496-1212) Establishment Name Address ID/FEI Business Operations SpecGx LLC 163205300 api manufacture(12496-1202, 12496-1204, 12496-1208, 12496-1212) , analysis(12496-1202, 12496-1204, 12496-1208, 12496-1212) Establishment Name Address ID/FEI Business Operations Veranova, L.P. 808176705 api manufacture(12496-1202, 12496-1204, 12496-1208, 12496-1212)