Label: OLPRUVA- sodium phenylbutyrate kit
-
NDC Code(s):
72542-000-01,
72542-002-01,
72542-003-01,
72542-200-02, view more72542-200-09, 72542-300-02, 72542-300-09, 72542-367-01, 72542-400-02, 72542-400-18, 72542-500-02, 72542-500-18, 72542-600-02, 72542-600-18, 72542-667-02, 72542-667-18
- Packager: Acer Therapeutics Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 22, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use OLPRUVATM safely and effectively. See full prescribing information for OLPRUVA.
OLPRUVA (sodium phenylbutyrate) for oral suspension
Initial U.S. Approval: 1996INDICATIONS AND USAGE
- OLPRUVA is a nitrogen-binding agent indicated as adjunctive therapy to standard of care, which includes dietary management, for the chronic management of adult and pediatric patients weighing 20 kg or greater and with a body surface area (BSA) of 1.2 m2 or greater, with urea cycle disorders (UCDs) involving deficiencies of carbamylphosphate synthetase (CPS), ornithine transcarbamylase (OTC), or argininosuccinic acid synthetase (AS). (1)
Limitations of Use:
OLPRUVA is not indicated for the treatment of acute hyperammonemia. (1)
DOSAGE AND ADMINISTRATION
- OLPRUVA treatment should be supervised by a healthcare provider experienced in the treatment of UCDs. For preparation and administration, see full prescribing information. (2.1, 2.4)
- The recommended dosage is 9.9 -13 g/m2/day. (2.1)
- Monitor plasma ammonia levels to determine the need for dosage adjustment. (2.2)
- Monitor patients for potential neurotoxicity. (2.2)
- For patients with hepatic impairment, start at the lower end of the recommended dosing range. (2.3)
DOSAGE FORMS AND STRENGTHS
- For oral suspension: 2 g, 3 g, 4 g, 5 g, 6 g, and 6.67 g of sodium phenylbutyrate as pellets in packets for reconstitution. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Neurotoxicity of Phenylacetate: Increased exposure to phenylacetate, the major metabolite of OLPRUVA, may be associated with neurotoxicity in patients with UCDs. Consider reducing the dose if neurotoxicity symptoms are present. (5.1)
- Hypokalemia: Renal excretion of phenylacetylglutamine may induce urinary loss of potassium. Monitor serum potassium during therapy and initiate appropriate treatment when necessary. (5.2)
- Conditions Associated with Edema: Calculate the total amount of sodium patients will be exposed to based on their body surface area. If a patient develops new-onset edema or worsening edema while on treatment, discontinue administration of OLPRUVA and initiate appropriate therapy. (5.3)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 3%) are menstrual dysfunction, decreased appetite, body odor and bad taste or taste aversion. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Acer Therapeutics Inc. at 1-844-600-2237 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Dosage Administration and Monitoring
2.3 Dosage Adjustment in Patients with Hepatic Impairment
2.4 Preparation and Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Neurotoxicity of Phenylacetate
5.2 Hypokalemia
5.3 Conditions Associated with Edema
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Potential for Other Drugs to Affect Ammonia
7.2 Potential for Other Drugs to Affect OLPRUVA
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
OLPRUVA is indicated as adjunctive therapy to standard of care, which includes dietary management, for the chronic management of adult and pediatric patients weighing 20 kg or greater and with a body surface area (BSA) of 1.2 m2 or greater, with urea cycle disorders (UCDs) involving deficiencies of carbamylphosphate synthetase (CPS), ornithine transcarbamylase (OTC), or argininosuccinic acid synthetase (AS).
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
OLPRUVA treatment should be supervised by a healthcare provider experienced in the treatment of urea cycle disorders.
The recommended dosage of OLPRUVA for patients with urea cycle disorders is 9.9 –13 g/m2/day orally. Divide the calculated total daily dose into three to six doses. Administer as three to six divided doses and take with food.
Round each individual dose of OLPRUVA to the nearest available dosage strength. The maximum dosage is 20 grams per day. Combine OLPRUVA with dietary protein restriction and, in some cases, amino acid supplementation (e.g., essential amino acids, arginine, citrulline, and protein-free calorie supplements).
If a dose is missed, take the missed dose as soon as possible on the same day.
2.2 Dosage Administration and Monitoring
Monitor plasma ammonia levels to determine the need for dosage adjustment. Adjust the OLPRUVA dosage to maintain the plasma ammonia level within the normal range for the patient's age, taking into consideration their clinical condition (e.g., nutritional requirements, protein intake, growth parameters, etc.).
Monitor patients for potential neurotoxicity and obtain measurements of plasma phenylacetate and phenylacetylglutamine levels [see Warnings and Precautions (5.1), Adverse Reactions (6)]. If neurologic symptoms (e.g., vomiting, nausea, headache, somnolence, or confusion) are present in the absence of high ammonia levels or other incurrent illnesses, consider reducing the dose of OLPRUVA.
2.3 Dosage Adjustment in Patients with Hepatic Impairment
For patients with hepatic impairment, start at the lower end of the recommended dosing range and maintain patients on the lowest dose necessary to control plasma ammonia levels [see Use in Specific Populations (8.7)].
2.4 Preparation and Administration Instructions
For oral administration only. Do not administer via gastrostomy or nasogastric tubes.
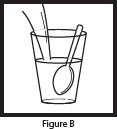
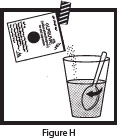
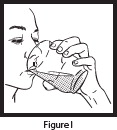
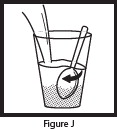
- Pour the entire contents of the Mix-Aid packet into approximately 4 ounces of water in a cup and stir, forming a suspension.
- Pour the entire contents of the OLPRUVA packet(s) into the suspension and stir.
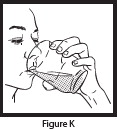
- Drink the entire suspension within 5 minutes after stirring to minimize dissolution of coating. After 30 minutes, the suspension should be discarded.
- Pour another 4 ounces of water into the cup and drink to make sure that any OLPRUVA remaining in the cup is consumed.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Neurotoxicity of Phenylacetate
Increased exposure to phenylacetate, the major metabolite of OLPRUVA, may be associated with neurotoxicity in patients with UCDs. In a study of adult cancer patients receiving intravenous phenylacetate, 250-300 mg/kg/day for 14 days, repeated at 4-week intervals, signs and symptoms of neurotoxicity, which were reversible upon discontinuation, were seen at plasma concentrations ≥ 3.5 mmol/L, and included somnolence, fatigue, and light headedness [see Adverse Reactions (6)]. OLPRUVA is not approved for intravenous use or for treatment of patients with cancer.
If symptoms of vomiting, nausea, headache, somnolence, or confusion are present in the absence of high ammonia levels or other intercurrent illnesses, consider reducing the dose of OLPRUVA [see Dosage and Administration (2.2)].
Phenylacetate caused neurotoxicity when given subcutaneously in rat pups [see Use in Specific Populations (8.4)].
5.2 Hypokalemia
Renal excretion of phenylacetylglutamine may induce urinary loss of potassium. Monitor serum potassium during therapy and initiate appropriate treatment when necessary.
5.3 Conditions Associated with Edema
OLPRUVA contains 124 mg (5.4 mmol) of sodium per gram of sodium phenylbutyrate (12.4% w/w) and the Mix-Aid contains 5 mg of sodium per packet, corresponding to 2.5 g (108 mmol) of sodium in the maximum daily dose of 20 g of OLPRUVA. In order to decide if administration of OLPRUVA is appropriate in patients with diseases that involve edema, such as heart failure, cirrhosis, or nephrosis, calculate the total amount of sodium patients will be exposed to based on their BSAs [see Dosage and Administration (2.1)]. If a patient develops new-onset edema or worsening edema while on treatment, discontinue administration of OLPRUVA and initiate appropriate therapy.
-
6 ADVERSE REACTIONS
The following adverse reactions associated with the use of sodium phenylbutyrate were identified in clinical studies or postmarketing reports. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Most common adverse reactions (incidence ≥ 3%) are amenorrhea or menstrual dysfunction (irregular menstrual cycles), decreased appetite, body odor and bad taste or taste aversion.
Less Common Clinical Adverse Reactions
Blood and lymphatic system disorders: aplastic anemia, ecchymoses
Cardiac disorders: arrhythmia
Gastrointestinal disorders: abdominal pain, gastritis, nausea and vomiting, constipation, rectal bleeding, peptic ulcer disease, pancreatitis
Metabolism and nutrition disorders: increased weight, edema
Nervous system disorders: syncope, headache
Psychiatric disorders: depression
Renal and urinary disorders: renal tubular acidosis
Skin and subcutaneous tissue disorders: rash
Laboratory Adverse Reactions
Blood and lymphatic system disorders: anemia, leukopenia and leukocytosis, thrombocytopenia, thrombocytosis
Hepatobiliary disorders: hyperbilirubinemia, increased blood alkaline phosphatase, increased transaminases
Metabolism and nutrition disorders: acidosis, alkalosis, hyperchloremia, hypophosphatemia, hyperuricemia, hyperphosphatemia, hypernatremia, hypokalemia, hypoalbuminemia, decreased total protein
Clinical Adverse Reactions with Use of Phenylacetate
Nervous system disorders: Neurotoxicity was reported in cancer patients receiving intravenous phenylacetate, the major metabolite of OLPRUVA (OLPRUVA is not approved for intravenous use or for treatment of patients with cancer). Signs and symptoms were predominately somnolence, fatigue, and dizziness (lightheadedness); less frequently reported were headache, dysgeusia, hypoacusis, disorientation, memory impairment, and exacerbation of a pre-existing neuropathy.
-
7 DRUG INTERACTIONS
7.1 Potential for Other Drugs to Affect Ammonia
7.2 Potential for Other Drugs to Affect OLPRUVA
Probenecid
Probenecid may inhibit renal excretion of the metabolites of OLPRUVA including phenylacetate and phenylacetylglutamine. Monitor patients for potential neurotoxicity and measure plasma phenylacetate and phenylacetylglutamine levels when probenecid is used concomitantly with OLPRUVA [see Dosage and Administration (2.2)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data with sodium phenylbutyrate use in pregnant women are insufficient to identify a drug associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Animal reproduction studies have not been conducted with sodium phenylbutyrate. Based on published animal data, phenylacetate may be neurotoxic to the developing brain (see Data).
There are serious risks to the mother and fetus associated with untreated urea cycle disorders during pregnancy which can result in serious morbidity and mortality to the mother and fetus (see Clinical Considerations).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Pregnancy is a time of increased metabolic demand which increases the risk for hyperammonemic episodes when metabolic demands are not met. Hyperammonemic episodes in pregnancy are associated with impaired cognition in the mother and an increased risk of maternal and fetal death.
8.2 Lactation
Risk Summary
There are no data on the presence of sodium phenylbutyrate and its metabolite in either human or animal milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for OLPRUVA and any potential adverse effects on the breastfed infant from OLPRUVA or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of OLPRUVA have been established as adjunctive therapy to the standard of care, which includes dietary management, in the chronic management of pediatric patients weighing 20 kg or greater and with a body surface area 1.2 m2 or greater, with urea cycle disorders (UCDs) involving deficiencies of carbamylphosphate synthetase (CPS), ornithine transcarbamylase (OTC), or argininosuccinic acid synthetase (AS).
OLPRUVA is not indicated for the treatment of acute hyperammonemia which can be a life-threatening medical emergency that requires rapid acting interventions to reduce plasma ammonia levels.
The sodium content of OLPRUVA has the potential to cause new-onset edema or worsening edema from salt and water retention, particularly in patients with underlying predisposing conditions [see Warnings and Precautions (5.3)].
OLPRUVA is not approved in pediatric patients weighing less than 20 kg or in pediatric patients weighing 20 kg or greater with a BSA of less than 1.2 m2.
Neurotoxicity has been observed in juvenile animals with phenylacetate exposure [see Warnings and Precautions (5.1)].
Juvenile Animal Toxicity Data
When given subcutaneously to neonatal rats, 190-474 mg/kg phenylacetate caused decreased proliferation and increased loss of neurons, and it reduced CNS myelin. Cerebral synapse maturation was retarded, and the number of functioning nerve terminals in the cerebrum was reduced, which resulted in impaired brain growth.
8.5 Geriatric Use
Clinical studies of OLPRUVA did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
8.6 Renal Impairment
No studies with OLPRUVA were conducted in subjects with renal impairment. Monitor plasma ammonia levels when starting patients with impaired renal function on OLPRUVA [see Clinical Pharmacology (12)].
8.7 Hepatic Impairment
No studies with OLPRUVA were conducted in subjects with hepatic impairment. Start at the lower end of the recommended dosing range and maintain patients with hepatic impairment on the lowest dose necessary to control plasma ammonia levels [see Clinical Pharmacology (12), Dosage and Administration (2.3)].
-
10 OVERDOSAGE
Overdoses of OLPRUVA exceeding ten-fold the maximum recommended dosage may produce emesis, CNS depression, metabolic acidosis with or without respiratory alkalosis, hypernatremia, hypokalemia, and hypophosphatemia. Symptoms of overdose overlap with those of acute hyperammonemia. If overdose occurs, discontinue OLPRUVA, monitor plasma phenylacetate and ammonia levels closely, and institute appropriate emergency management, which may include hemodialysis, continuous veno-venous hemofiltration (CVVH) or extracorporeal membrane oxygenation (ECMO).
-
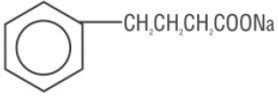
11 DESCRIPTION
OLPRUVA (sodium phenylbutyrate) for oral suspension is a nitrogen binding agent. Sodium phenylbutyrate is a white to yellowish-white powder. It is freely soluble in water and in methanol, and practically insoluble in acetone and diethyl ether. It is known chemically as sodium 4-phenylbutyrate with a molecular weight of 186.19 and molecular formula C10H11NaO2.
Structural Formula:
OLPRUVA is supplied in dosage envelopes containing 2 g (equivalent to 1.75 g phenylbutyrate), 3 g (equivalent to 2.63 g phenylbutyrate), 4 g (equivalent to 3.51 g phenylbutyrate), 5 g (equivalent to 4.38 g phenylbutyrate), 6 g (equivalent to 5.26 g phenylbutyrate), and 6.67 g (equivalent to 5.85 g phenylbutyrate) of sodium phenylbutyrate in one or two packets. OLPRUVA is a polymer coated formulation which contains the following inactive ingredients: amino methacrylate copolymer, hypromellose, microcrystalline cellulose, polyethylene glycol 6000, silicon dioxide, and talc.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Sodium phenylbutyrate is a pro-drug and is metabolized to phenylacetate. Phenylacetate is a metabolically active compound that conjugates with glutamine via acetylation to form phenylacetylglutamine. Phenylacetylglutamine is excreted by the kidneys, hence providing an alternate vehicle for waste nitrogen excretion.
12.2 Pharmacodynamics
In patients with urea cycle disorders, sodium phenylbutyrate decreased elevated plasma ammonia and glutamine levels.
12.3 Pharmacokinetics
The pharmacokinetics of phenylbutyrate and its metabolite phenylacetate were characterized in healthy adult subjects following a single oral administration of OLPRUVA (5 g of sodium phenylbutyrate) with suspension agent under fasted and fed conditions.
Absorption
The pharmacokinetic parameters for the maximum plasma concentration (Cmax) and area under the plasma concentration-time curve (AUC) of phenylbutyrate and phenylacetate under fasted conditions are summarized in Table 1.
Table 1 Cmax and AUC of Phenylbutyrate and Phenylacetate Following a Single Oral Dose Administration of OLPRUVA (5 g) in Healthy Subjects Under Fasted Conditions PK Parameters Phenylbutyrate Results
(Mean ± SD)Phenylacetate Results
(Mean ± SD)Cmax (μg/mL) 229 ± 48 39 ± 14 AUCinf (hr•μg/mL) 510 ± 129 183 ± 76 Effect of Food
Compared to those under fasted conditions, phenylbutyrate Cmax was decreased by 50% and AUCinf decreased by 39% when OLPRUVA was administered with a high-fat meal (total 980 calories with 55% fat). For the metabolite phenylacetate, Cmax decreased by 32% and AUCinf decreased by 29% with a high-fat meal compared to fasted conditions.
Distribution
The apparent volume of distribution of phenylbutyrate was 7.2 L under fasted conditions.
Elimination
The mean half-life of phenylbutyrate was 0.5 hours under fasted conditions. The mean half-life of phenylacetate was 1.2 hours under fasted conditions.
Metabolism
Following oral administration, sodium phenylbutyrate is metabolized by β-oxidation into phenylacetate which is converted to its coenzyme A ester, phenylacetyl-coenzyme A and further conjugated with glutamine to form phenylacetylglutamine. Phenylacetylglutamine is excreted by the kidneys. The major sites for metabolism of sodium phenylbutyrate are the liver and kidneys. Phenylacetate is also hydrolyzed by esterases in liver and blood.
- 13 NONCLINICAL TOXICOLOGY
-
16 HOW SUPPLIED/STORAGE AND HANDLING
OLPRUVA (sodium phenylbutyrate) for oral suspension is available in dosage strengths of 2 g, 3 g, 4 g, 5 g, 6 g, and 6.67 g of sodium phenylbutyrate as white to off-white pellets. Each dose is packaged in a dosage envelope containing one or two packets of sodium phenylbutyrate for oral suspension and a suspending agent packet (labeled as Mix-Aid). A 30-day supply of OLPRUVA is provided in a kit containing 90 dosage envelopes.
Table 2 OLPRUVA Available Dosage Strengths Dosage Strength OLPRUVA packet(s) in each envelope Envelope NDC Kit NDC 2 g one 2 g packet (NDC 72542-002-01) 72542-200-02 72542-200-09 3 g one 3 g packet (NDC 72542-003-01) 72542-300-02 72542-300-09 4 g two 2 g packets 72542-400-02 72542-400-18 5 g one 2 g packet and one 3 g packet 72542-500-02 72542-500-18 6 g two 3 g packets 72542-600-02 72542-600-18 6.67 g one 3 g packet and one 3.67 g packet (NDC 72542-367-01) 72542-667-02 72542-667-18 Store OLPRUVA at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Neurotoxicity
Advise the patient or caregiver that neurotoxicity may occur during OLPRUVA treatment. Inform the patient or caregiver of the signs and symptoms of this risk and to contact the healthcare provider immediately if signs and symptoms occur [see Warnings and Precautions (5.1)].
Preparation and Administration
Inform the patient or caregiver that the OLPRUVA packet(s) must be mixed with the prepared Mix-Aid suspension and to drink the entire suspension within 5 minutes after stirring to minimize dissolution of coating. After 30 minutes, the suspension should be discarded [see Dosage and Administration (2.3)].
Inform the patient or caregiver that if a dose is missed, take the missed dose as soon as possible on the same day [see Dosage and Administration (2.1)].
Manufactured for:
Acer Therapeutics Inc.
300 Washington St.
Newton, MA 02458
For more information call Acer Therapeutics Inc. at 1-844-600-2237.
© 2022 Acer Therapeutics Inc. All rights reserved.65003012
-
PATIENT PACKAGE INSERT
-
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
OLPRUVA™ (ol proo vah)
(sodium phenylbutyrate)
for oral suspensionRead this Instructions for Use before taking OLPRUVA oral suspension and each time you get a refill. There may be new information. This Instructions for Use does not take the place of talking to your healthcare provider about your medical condition or treatment. Talk to your healthcare provider or pharmacist if you have any questions about how to take a dose of OLPRUVA.
This Instructions for Use contains information on how to prepare and take 1 dose of OLPRUVA.
Supplies needed to take 1 dose of OLPRUVA as prescribed:
- One dosage envelope containing OLPRUVA and Mix-Aid packets. The contents of 1 envelope equals 1 full dose.
- An open drinking cup
- A spoon
- Water
How should I store OLPRUVA?
- Store OLPRUVA at room temperature between 68°F and 77°F (20°C and 25°C).
- Keep OLPRUVA and all medicines out of the reach of children.
Manufactured for:
Acer Therapeutics Inc.
300 Washington St.
Newton, MA 02458
For more information, go to www.OLPRUVA.com or call Acer Therapeutics Inc. at 1-844-600-2237.
© 2022 Acer Therapeutics Inc. All rights reserved.This Instructions for Use has been approved by the U.S. Food and Drug Administration. Issued: 12/2022
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel – Mix-Aid Label
Tear or Cut
Mix-Aid™
Suspending agent for use with
OLPRUVA™ (sodium phenylbutyrate)
for oral suspensionDirections: Mix full contents of packet
per patient instructions for use.The contents of this packet will NOT
dissolve but will make the water thicker
to suspend OLPRUVA™.NET WT. 0.11 OZ (3.1 g)
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 2 g Carton Label
NDC 72542-200-09
OLPRUVA™
(sodium phenylbutyrate)
for oral suspension2g
Rx Only
Recommended Dosage: See Prescribing Information.
Kit contains 90 dosage envelopes. Each envelope contains one OLPRUVA™ 2 gram
packet and one Mix-Aid™ packet.Each 2 g of sodium phenylbutyrate is equivalent to 1.75 g of phenylbutyrate.
See accompanying patient instructions for use.
Store at 20°C to 25°C (68°F to 77°F); excursions permitted
between 15°C and 30°C (59°F and 86°F) [See USP Controlled
Room Temperature].Keep out of reach of children
acertherapeutics
-
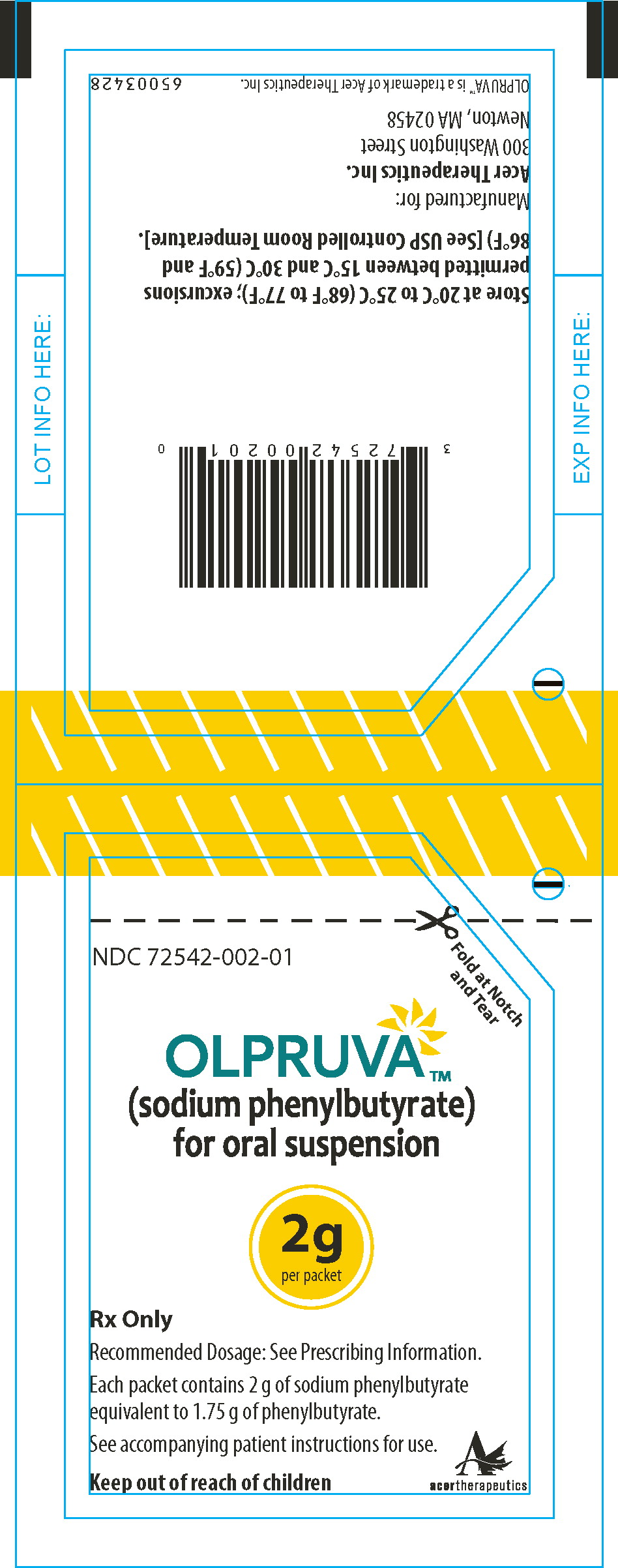
PRINCIPAL DISPLAY PANEL
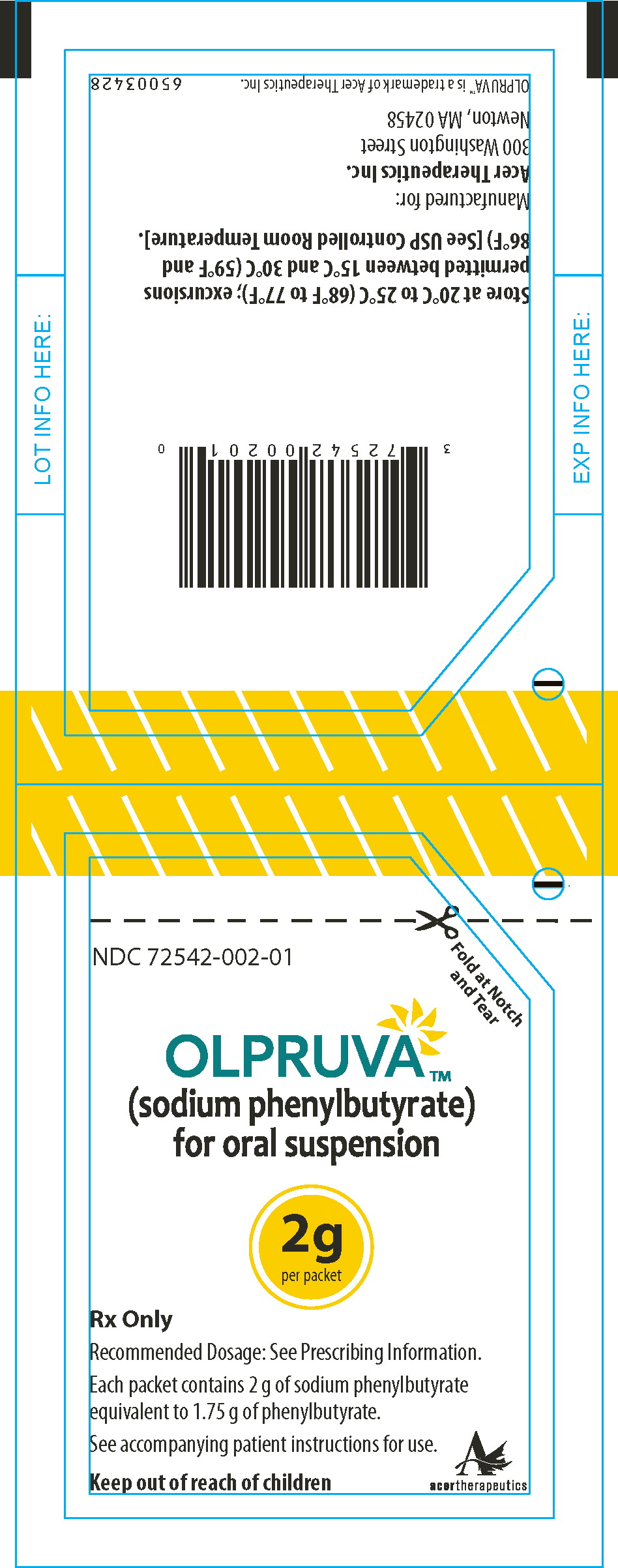
Principal Display Panel - 2 g Pouch Label
Fold at Notch
and TearNDC 72542-002-01
OLPRUVA™
(sodium phenylbutyrate)
for oral suspension2g
per packetRx Only
Recommended Dosage: See Prescribing Information.
Each packet contains 2 g of sodium phenylbutyrate
equivalent to 1.75 g of phenylbutyrate.See accompanying patient instructions for use.
Keep out of reach of children
acertherapeutics
Store at 20°C to 25°C (68°F to 77°F); excursions permitted
between 15°C and 30°C (59°F and 86°F) [See USP Controlled
Room Temperature]. -
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 2 g Envelope Label
NDC 72542-200-02
OLPRUVA™
(sodium phenylbutyrate)
for oral suspension2g
Rx Only
Keep out of reach
of childrenRecommended Dosage: See Prescribing Information.
Each envelope contains one OLPRUVA™ 2 gram packet and one Mix-Aid™ packet.
Each 2 g of sodium phenylbutyrate is equivalent to 1.75 g of phenylbutyrate.
acertherapeutics
Store at 20°C to 25°C (68°F to 77°F); excursions permitted
between 15°C and 30°C (59°F and 86°F) [See USP Controlled
Room Temperature]. -
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 3 g Carton Label
NDC 72542-300-09
OLPRUVA™
(sodium phenylbutyrate)
for oral suspension3g
Rx Only
Recommended Dosage: See Prescribing Information.
Kit contains 90 dosage envelopes. Each envelope contains one OLPRUVA™ 3 gram
packet and one Mix-Aid™ packet.Each 3 g of sodium phenylbutyrate is equivalent to 2.63 g of phenylbutyrate.
See accompanying patient instructions for use.
Store at 20°C to 25°C (68°F to 77°F); excursions permitted
between 15°C and 30°C (59°F and 86°F) [See USP Controlled
Room Temperature].Keep out of reach of children
acertherapeutics
-
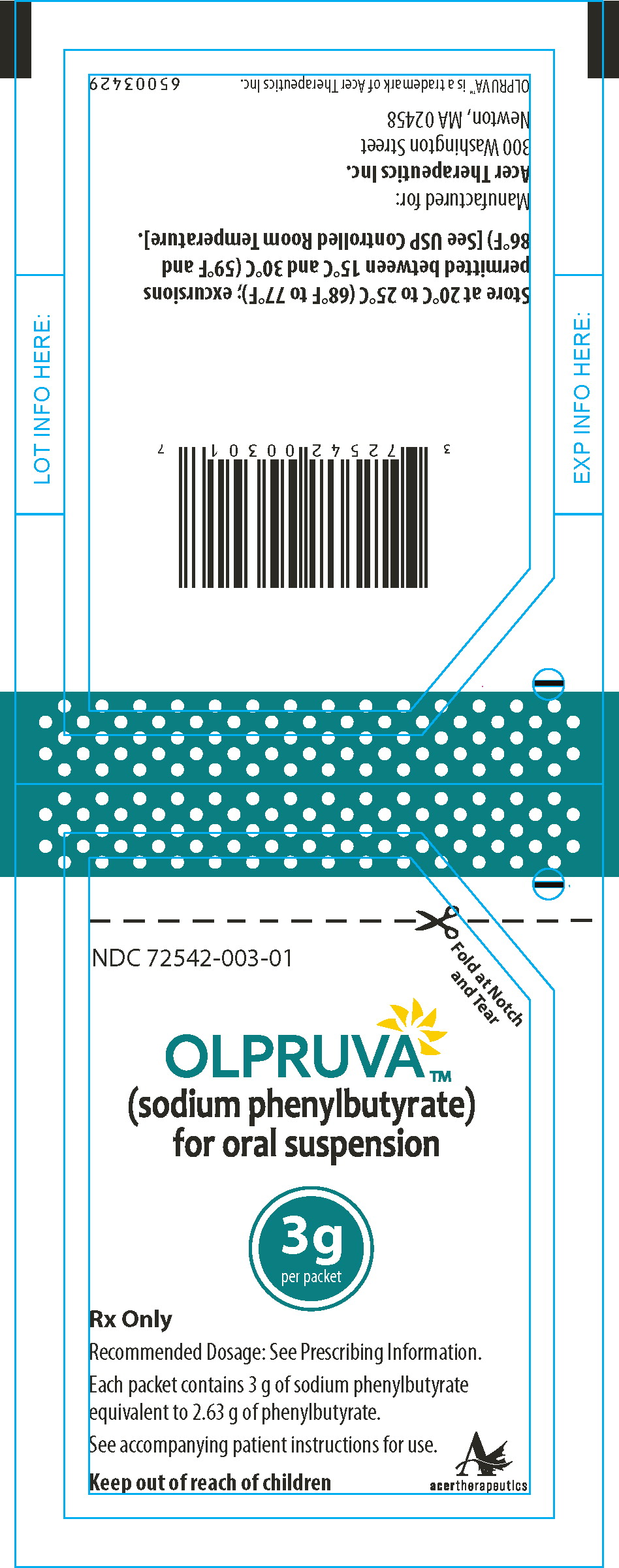
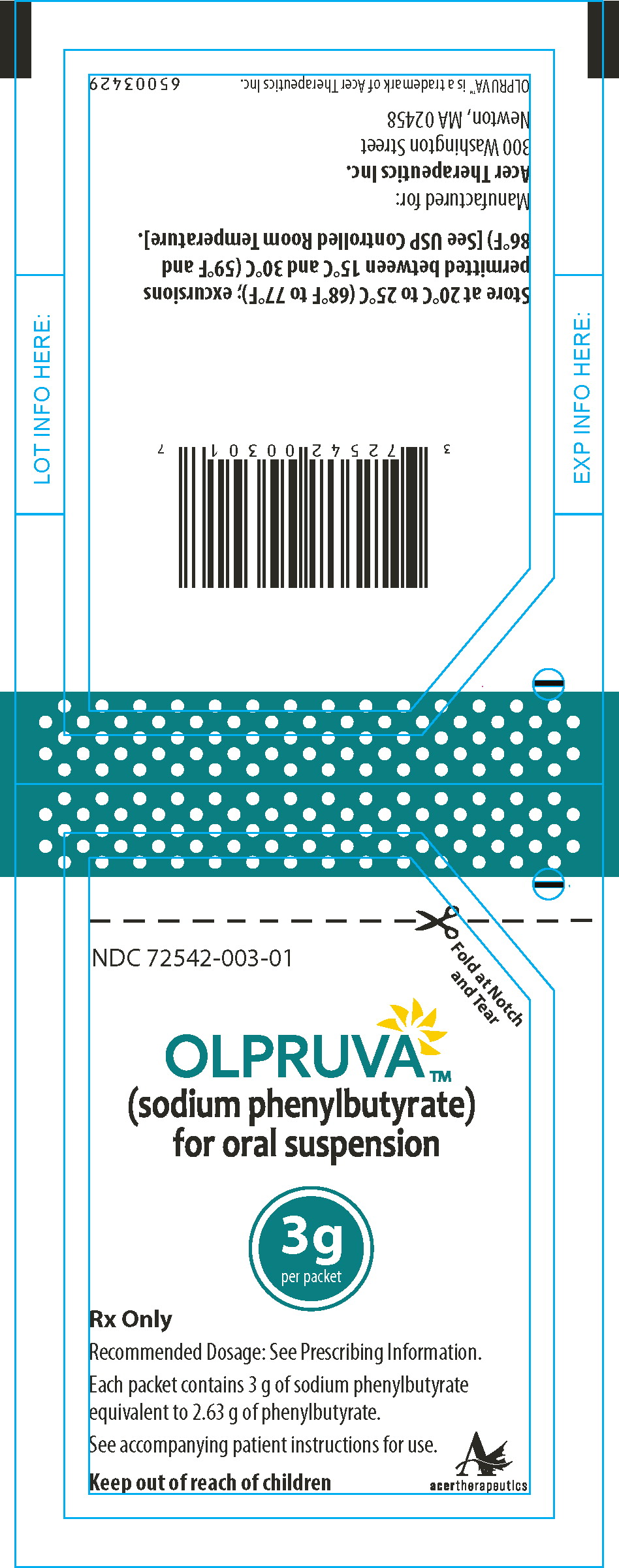
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 3 g Pouch Label
Fold at Notch
and TearNDC 72542-003-01
OLPRUVA™
(sodium phenylbutyrate)
for oral suspension3g
per packetRx Only
Recommended Dosage: See Prescribing Information.
Each packet contains 3 g of sodium phenylbutyrate
equivalent to 2.63 g of phenylbutyrate.See accompanying patient instructions for use.
Keep out of reach of children
acertherapeutics
Store at 20°C to 25°C (68°F to 77°F); excursions permitted
between 15°C and 30°C (59°F and 86°F) [See USP Controlled
Room Temperature]. -
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 3 g Envelope Label
NDC 72542-300-02
OLPRUVA™
(sodium phenylbutyrate)
for oral suspension3g
Rx Only
Keep out of reach
of childrenRecommended Dosage: See Prescribing Information.
Each envelope contains one OLPRUVA™ 3 gram packet and one Mix-Aid™ packet.
Each 3 g of sodium phenylbutyrate is equivalent to 2.63 g of phenylbutyrate.
acertherapeutics
Store at 20°C to 25°C (68°F to 77°F); excursions permitted
between 15°C and 30°C (59°F and 86°F) [See USP Controlled
Room Temperature]. -
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 4 g Carton Label
NDC 72542-400-18
OLPRUVA™
(sodium phenylbutyrate)
for oral suspension4g
Rx Only
Recommended Dosage: See Prescribing Information.
Kit contains 90 dosage envelopes. Each envelope contains two OLPRUVA™ 2 gram
packets and one Mix-Aid™ packet.Each 4 g of sodium phenylbutyrate is equivalent to 3.51 g of phenylbutyrate.
See accompanying patient instructions for use.
Store at 20°C to 25°C (68°F to 77°F); excursions permitted
between 15°C and 30°C (59°F and 86°F) [See USP Controlled
Room Temperature].Keep out of reach of children
acertherapeutics
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 4 g Envelope Label
NDC 72542-400-02
OLPRUVA™
(sodium phenylbutyrate)
for oral suspension4g
Rx Only
Keep out of reach
of childrenRecommended Dosage: See Prescribing Information.
Each envelope contains two OLPRUVA™ 2 gram packets and one Mix-Aid™ packet.
Each 4 g of sodium phenylbutyrate is equivalent to 3.51 g of phenylbutyrate.
acertherapeutics
Store at 20°C to 25°C (68°F to 77°F); excursions permitted
between 15°C and 30°C (59°F and 86°F) [See USP Controlled
Room Temperature]. -
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 5 g Carton Label
NDC 72542-500-18
OLPRUVA™
(sodium phenylbutyrate)
for oral suspension5g
Rx Only
Recommended Dosage: See Prescribing Information.
Kit contains 90 dosage envelopes. Each envelope contains one OLPRUVA™ 2 gram
packet, one OLPRUVA™ 3 gram packet, and one Mix-Aid™ packet.Each 5 g of sodium phenylbutyrate is equivalent to 4.38 g of phenylbutyrate.
See accompanying patient instructions for use.
Store at 20°C to 25°C (68°F to 77°F); excursions permitted
between 15°C and 30°C (59°F and 86°F) [See USP Controlled
Room Temperature].Keep out of reach of children
acertherapeutics
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 5 g Envelope Label
NDC 72542-500-02
OLPRUVA™
(sodium phenylbutyrate)
for oral suspension5g
Rx Only
Keep out of reach
of childrenRecommended Dosage: See Prescribing Information.
Each envelope contains one OLPRUVA™ 2 gram packet, one OLPRUVA™ 3 gram
packet, and one Mix-Aid™ packet.
Each 5 g of sodium phenylbutyrate is equivalent to 4.38 g of phenylbutyrate.
acertherapeutics
Store at 20°C to 25°C (68°F to 77°F); excursions permitted
between 15°C and 30°C (59°F and 86°F) [See USP Controlled
Room Temperature]. -
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 6 g Carton Label
NDC 72542-600-18
OLPRUVA™
(sodium phenylbutyrate)
for oral suspension6g
Rx Only
Recommended Dosage: See Prescribing Information.
Kit contains 90 dosage envelopes. Each envelope contains two OLPRUVA™ 3 gram
packets and one Mix-Aid™ packet.Each 6 g of sodium phenylbutyrate is equivalent to 5.26 g of phenylbutyrate.
See accompanying patient instructions for use.
Store at 20°C to 25°C (68°F to 77°F); excursions permitted
between 15°C and 30°C (59°F and 86°F) [See USP Controlled
Room Temperature].Keep out of reach of children
acertherapeutics
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 6 g Envelope Label
NDC 72542-600-02
OLPRUVA™
(sodium phenylbutyrate)
for oral suspension6g
Rx Only
Keep out of reach
of childrenRecommended Dosage: See Prescribing Information.
Each envelope contains two OLPRUVA™ 3 gram packets and one Mix-Aid™ packet.
Each 6 g of sodium phenylbutyrate is equivalent to 5.26 g of phenylbutyrate.
acertherapeutics
Store at 20°C to 25°C (68°F to 77°F); excursions permitted
between 15°C and 30°C (59°F and 86°F) [See USP Controlled
Room Temperature]. -
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 6.67 g Carton Label
NDC 72542-667-18
OLPRUVA™
(sodium phenylbutyrate)
for oral suspension6.67g
Rx Only
Recommended Dosage: See Prescribing Information.
Kit contains 90 dosage envelopes. Each envelope contains one OLPRUVA™ 3 gram
packet, one OLPRUVA™ 3.67 gram packet, and one Mix-Aid™ packet.Each 6.67 g of sodium phenylbutyrate is equivalent to 5.85 g of phenylbutyrate.
See accompanying patient instructions for use.
Store at 20°C to 25°C (68°F to 77°F); excursions permitted
between 15°C and 30°C (59°F and 86°F) [See USP Controlled
Room Temperature].Keep out of reach of children
acertherapeutics
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 6.67 g Envelope Label
NDC 72542-667-02
OLPRUVA™
(sodium phenylbutyrate)
for oral suspension6.67g
Rx Only
Keep out of reach
of childrenRecommended Dosage: See Prescribing Information.
Each envelope contains one OLPRUVA™ 3 gram packet, one OLPRUVA™ 3.67 gram
packet, and one Mix-Aid™ packet.
Each 6.67 g of sodium phenylbutyrate is equivalent to 5.85 g of phenylbutyrate.
acertherapeutics
Store at 20°C to 25°C (68°F to 77°F); excursions permitted
between 15°C and 30°C (59°F and 86°F) [See USP Controlled
Room Temperature]. -
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 3.67 g Pouch Label
Fold at Notch
and TearNDC 72542-367-01
OLPRUVA™
(sodium phenylbutyrate)
for oral suspension3.67g
per packetRx Only
Recommended Dosage: See Prescribing Information.
Each packet contains 3.67 g of sodium phenylbutyrate
equivalent to 3.22 g of phenylbutyrate.See accompanying patient instructions for use.
Keep out of reach of children
acertherapeutics
Store at 20°C to 25°C (68°F to 77°F); excursions permitted
between 15°C and 30°C (59°F and 86°F) [See USP Controlled
Room Temperature]. -
INGREDIENTS AND APPEARANCE
OLPRUVA
sodium phenylbutyrate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72542-200 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72542-200-09 90 in 1 CARTON 12/22/2022 1 NDC:72542-200-02 1 in 1 BOX; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 PACKET 1 Part 2 1 PACKET 1 Part 1 of 2 OLPRUVA
sodium phenylbutyrate for suspensionProduct Information Item Code (Source) NDC:72542-002 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength sodium phenylbutyrate (UNII: NT6K61736T) (phenylbutyric acid - UNII:7WY7YBI87E) sodium phenylbutyrate 2 g Inactive Ingredients Ingredient Name Strength Polyethylene glycol 6000 (UNII: 30IQX730WE) Silicon Dioxide (UNII: ETJ7Z6XBU4) Microcrystalline Cellulose (UNII: OP1R32D61U) Hypromellose 2910 (5 MPA.S) (UNII: R75537T0T4) Talc (UNII: 7SEV7J4R1U) DIMETHYLAMINOETHYL METHACRYLATE - BUTYL METHACRYLATE - METHYL METHACRYLATE COPOLYMER (UNII: 905HNO1SIH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72542-002-01 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214860 12/22/2022 Part 2 of 2 MIX-AID
starch, corn for suspensionProduct Information Item Code (Source) NDC:72542-000 Route of Administration ORAL Inactive Ingredients Ingredient Name Strength Starch, Corn (UNII: O8232NY3SJ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72542-000-01 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214860 12/22/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214860 12/22/2022 OLPRUVA
sodium phenylbutyrate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72542-300 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72542-300-09 90 in 1 CARTON 12/22/2022 1 NDC:72542-300-02 1 in 1 BOX; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 PACKET 1 Part 2 1 PACKET 1 Part 1 of 2 OLPRUVA
sodium phenylbutyrate for suspensionProduct Information Item Code (Source) NDC:72542-003 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength sodium phenylbutyrate (UNII: NT6K61736T) (phenylbutyric acid - UNII:7WY7YBI87E) sodium phenylbutyrate 3 g Inactive Ingredients Ingredient Name Strength Polyethylene glycol 6000 (UNII: 30IQX730WE) Silicon Dioxide (UNII: ETJ7Z6XBU4) Microcrystalline Cellulose (UNII: OP1R32D61U) Hypromellose 2910 (5 MPA.S) (UNII: R75537T0T4) Talc (UNII: 7SEV7J4R1U) DIMETHYLAMINOETHYL METHACRYLATE - BUTYL METHACRYLATE - METHYL METHACRYLATE COPOLYMER (UNII: 905HNO1SIH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72542-003-01 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214860 12/22/2022 Part 2 of 2 MIX-AID
starch, corn for suspensionProduct Information Item Code (Source) NDC:72542-000 Route of Administration ORAL Inactive Ingredients Ingredient Name Strength Starch, Corn (UNII: O8232NY3SJ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72542-000-01 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214860 12/22/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214860 12/22/2022 OLPRUVA
sodium phenylbutyrate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72542-400 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72542-400-18 90 in 1 CARTON 12/22/2022 1 NDC:72542-400-02 1 in 1 BOX; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 2 PACKET 2 Part 2 1 PACKET 1 Part 1 of 2 OLPRUVA
sodium phenylbutyrate for suspensionProduct Information Item Code (Source) NDC:72542-002 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength sodium phenylbutyrate (UNII: NT6K61736T) (phenylbutyric acid - UNII:7WY7YBI87E) sodium phenylbutyrate 2 g Inactive Ingredients Ingredient Name Strength Polyethylene glycol 6000 (UNII: 30IQX730WE) Silicon Dioxide (UNII: ETJ7Z6XBU4) Microcrystalline Cellulose (UNII: OP1R32D61U) Hypromellose 2910 (5 MPA.S) (UNII: R75537T0T4) Talc (UNII: 7SEV7J4R1U) DIMETHYLAMINOETHYL METHACRYLATE - BUTYL METHACRYLATE - METHYL METHACRYLATE COPOLYMER (UNII: 905HNO1SIH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72542-002-01 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214860 12/22/2022 Part 2 of 2 MIX-AID
starch, corn for suspensionProduct Information Item Code (Source) NDC:72542-000 Route of Administration ORAL Inactive Ingredients Ingredient Name Strength Starch, Corn (UNII: O8232NY3SJ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72542-000-01 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214860 12/22/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214860 12/22/2022 OLPRUVA
sodium phenylbutyrate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72542-500 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72542-500-18 90 in 1 CARTON 12/22/2022 1 NDC:72542-500-02 1 in 1 BOX; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 PACKET 1 Part 2 1 PACKET 1 Part 3 1 PACKET 1 Part 1 of 3 OLPRUVA
sodium phenylbutyrate for suspensionProduct Information Item Code (Source) NDC:72542-002 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength sodium phenylbutyrate (UNII: NT6K61736T) (phenylbutyric acid - UNII:7WY7YBI87E) sodium phenylbutyrate 2 g Inactive Ingredients Ingredient Name Strength Polyethylene glycol 6000 (UNII: 30IQX730WE) Silicon Dioxide (UNII: ETJ7Z6XBU4) Microcrystalline Cellulose (UNII: OP1R32D61U) Hypromellose 2910 (5 MPA.S) (UNII: R75537T0T4) Talc (UNII: 7SEV7J4R1U) DIMETHYLAMINOETHYL METHACRYLATE - BUTYL METHACRYLATE - METHYL METHACRYLATE COPOLYMER (UNII: 905HNO1SIH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72542-002-01 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214860 12/22/2022 Part 2 of 3 OLPRUVA
sodium phenylbutyrate for suspensionProduct Information Item Code (Source) NDC:72542-003 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength sodium phenylbutyrate (UNII: NT6K61736T) (phenylbutyric acid - UNII:7WY7YBI87E) sodium phenylbutyrate 3 g Inactive Ingredients Ingredient Name Strength Polyethylene glycol 6000 (UNII: 30IQX730WE) Silicon Dioxide (UNII: ETJ7Z6XBU4) Microcrystalline Cellulose (UNII: OP1R32D61U) Hypromellose 2910 (5 MPA.S) (UNII: R75537T0T4) Talc (UNII: 7SEV7J4R1U) DIMETHYLAMINOETHYL METHACRYLATE - BUTYL METHACRYLATE - METHYL METHACRYLATE COPOLYMER (UNII: 905HNO1SIH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72542-003-01 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214860 12/22/2022 Part 3 of 3 MIX-AID
starch, corn for suspensionProduct Information Item Code (Source) NDC:72542-000 Route of Administration ORAL Inactive Ingredients Ingredient Name Strength Starch, Corn (UNII: O8232NY3SJ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72542-000-01 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214860 12/22/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214860 12/22/2022 OLPRUVA
sodium phenylbutyrate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72542-600 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72542-600-18 90 in 1 CARTON 12/22/2022 1 NDC:72542-600-02 1 in 1 BOX; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 2 PACKET 2 Part 2 1 PACKET 1 Part 1 of 2 OLPRUVA
sodium phenylbutyrate for suspensionProduct Information Item Code (Source) NDC:72542-003 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength sodium phenylbutyrate (UNII: NT6K61736T) (phenylbutyric acid - UNII:7WY7YBI87E) sodium phenylbutyrate 3 g Inactive Ingredients Ingredient Name Strength Polyethylene glycol 6000 (UNII: 30IQX730WE) Silicon Dioxide (UNII: ETJ7Z6XBU4) Microcrystalline Cellulose (UNII: OP1R32D61U) Hypromellose 2910 (5 MPA.S) (UNII: R75537T0T4) Talc (UNII: 7SEV7J4R1U) DIMETHYLAMINOETHYL METHACRYLATE - BUTYL METHACRYLATE - METHYL METHACRYLATE COPOLYMER (UNII: 905HNO1SIH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72542-003-01 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214860 12/22/2022 Part 2 of 2 MIX-AID
starch, corn for suspensionProduct Information Item Code (Source) NDC:72542-000 Route of Administration ORAL Inactive Ingredients Ingredient Name Strength Starch, Corn (UNII: O8232NY3SJ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72542-000-01 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214860 12/22/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214860 12/22/2022 OLPRUVA
sodium phenylbutyrate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72542-667 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72542-667-18 90 in 1 CARTON 12/22/2022 1 NDC:72542-667-02 1 in 1 BOX; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 PACKET 1 Part 2 1 PACKET 1 Part 3 1 PACKET 1 Part 1 of 3 OLPRUVA
sodium phenylbutyrate for suspensionProduct Information Item Code (Source) NDC:72542-003 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength sodium phenylbutyrate (UNII: NT6K61736T) (phenylbutyric acid - UNII:7WY7YBI87E) sodium phenylbutyrate 3 g Inactive Ingredients Ingredient Name Strength Polyethylene glycol 6000 (UNII: 30IQX730WE) Silicon Dioxide (UNII: ETJ7Z6XBU4) Microcrystalline Cellulose (UNII: OP1R32D61U) Hypromellose 2910 (5 MPA.S) (UNII: R75537T0T4) Talc (UNII: 7SEV7J4R1U) DIMETHYLAMINOETHYL METHACRYLATE - BUTYL METHACRYLATE - METHYL METHACRYLATE COPOLYMER (UNII: 905HNO1SIH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72542-003-01 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214860 12/22/2022 Part 2 of 3 OLPRUVA
sodium phenylbutyrate for suspensionProduct Information Item Code (Source) NDC:72542-367 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength sodium phenylbutyrate (UNII: NT6K61736T) (phenylbutyric acid - UNII:7WY7YBI87E) sodium phenylbutyrate 3.67 g Inactive Ingredients Ingredient Name Strength Polyethylene glycol 6000 (UNII: 30IQX730WE) Silicon Dioxide (UNII: ETJ7Z6XBU4) Microcrystalline Cellulose (UNII: OP1R32D61U) Hypromellose 2910 (5 MPA.S) (UNII: R75537T0T4) Talc (UNII: 7SEV7J4R1U) DIMETHYLAMINOETHYL METHACRYLATE - BUTYL METHACRYLATE - METHYL METHACRYLATE COPOLYMER (UNII: 905HNO1SIH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72542-367-01 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214860 12/22/2022 Part 3 of 3 MIX-AID
starch, corn for suspensionProduct Information Item Code (Source) NDC:72542-000 Route of Administration ORAL Inactive Ingredients Ingredient Name Strength Starch, Corn (UNII: O8232NY3SJ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72542-000-01 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214860 12/22/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214860 12/22/2022 Labeler - Acer Therapeutics Inc. (079311509) Establishment Name Address ID/FEI Business Operations CH Chemie Uetikon GmbH 340717289 API MANUFACTURE(72542-200, 72542-300, 72542-400, 72542-500, 72542-600, 72542-667, 72542-002, 72542-003, 72542-367) Establishment Name Address ID/FEI Business Operations EMSL Analytical, Inc. DBA MPL Laboratories 080265475 ANALYSIS(72542-200, 72542-300, 72542-400, 72542-500, 72542-600, 72542-667, 72542-002, 72542-003, 72542-367) Establishment Name Address ID/FEI Business Operations Glatt Air Techniques Inc. 790220628 MANUFACTURE(72542-200, 72542-300, 72542-400, 72542-500, 72542-600, 72542-667, 72542-002, 72542-003, 72542-367) Establishment Name Address ID/FEI Business Operations Sharp Clinical Services LLC 079209266 PACK(72542-200, 72542-300, 72542-400, 72542-500, 72542-600, 72542-667, 72542-002, 72542-003, 72542-367)