Label: LIDOCAINE HYDROCHLORIDE injection, solution
- NDC Code(s): 55150-161-02, 55150-162-05, 55150-163-30, 55150-164-02, view more
- Packager: Eugia US LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 11, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONFor Infiltration and Nerve Block Including Caudal and Epidural Use. Preservative-Free - Rx only
-
DESCRIPTIONLidocaine hydrochloride injection, USP is sterile, nonpyrogenic, aqueous solution that contains a local anesthetic agent and is administered parenterally by injection. See INDICATIONS AND USAGE ...
-
CLINICAL PHARMACOLOGYMechanism of Action - Lidocaine hydrochloride stabilizes the neuronal membrane by inhibiting the ionic fluxes required for the initiation and conduction of impulses thereby effecting local ...
-
INDICATIONS AND USAGELidocaine hydrochloride injection is indicated for production of local or regional anesthesia by infiltration techniques such as percutaneous injection and intravenous regional anesthesia by ...
-
CONTRAINDICATIONSLidocaine hydrochloride is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type.
-
WARNINGSLIDOCAINE HYDROCHLORIDE INJECTION FOR INFILTRATION AND NERVE BLOCK SHOULD BE EMPLOYED ONLY BY CLINICIANS WHO ARE WELL VERSED IN DIAGNOSIS AND MANAGEMENT OF DOSE-RELATED TOXICITY AND OTHER ACUTE ...
-
PRECAUTIONSGeneral - The safety and effectiveness of lidocaine hydrochloride depend on proper dosage, correct technique, adequate precautions, and readiness for emergencies. Standard textbooks should be ...
-
ADVERSE REACTIONSSystemic - Adverse experiences following the administration of lidocaine hydrochloride are similar in nature to those observed with other amide local anesthetic agents. These adverse ...
-
OVERDOSAGEAcute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use of local anesthetics or to unintended subarachnoid injection of local ...
-
DOSAGE AND ADMINISTRATIONTable 1 (Recommended Dosages) summarizes the recommended volumes and concentrations of lidocaine hydrochloride injection for various types of anesthetic procedures. The dosages suggested in this ...
-
MAXIMUM RECOMMENDED DOSAGESAdults - For normal healthy adults, the maximum individual dose should not exceed 4.5 mg/kg (2 mg/lb) of body weight, and in general it is recommended that the maximum total dose does not ...
-
STERILIZATION, STORAGE AND TECHNICAL PROCEDURESDisinfecting agents containing heavy metals, which cause release of respective ions (mercury, zinc, copper, etc.) should not be used for skin or mucous membrane disinfection as they have been ...
-
HOW SUPPLIEDLidocaine Hydrochloride Injection, USP is supplied as follows: Lidocaine Hydrochloride Injection USP, 1% (10 mg/mL) 2 mL Single-Dose Vials in a Carton of ...
-
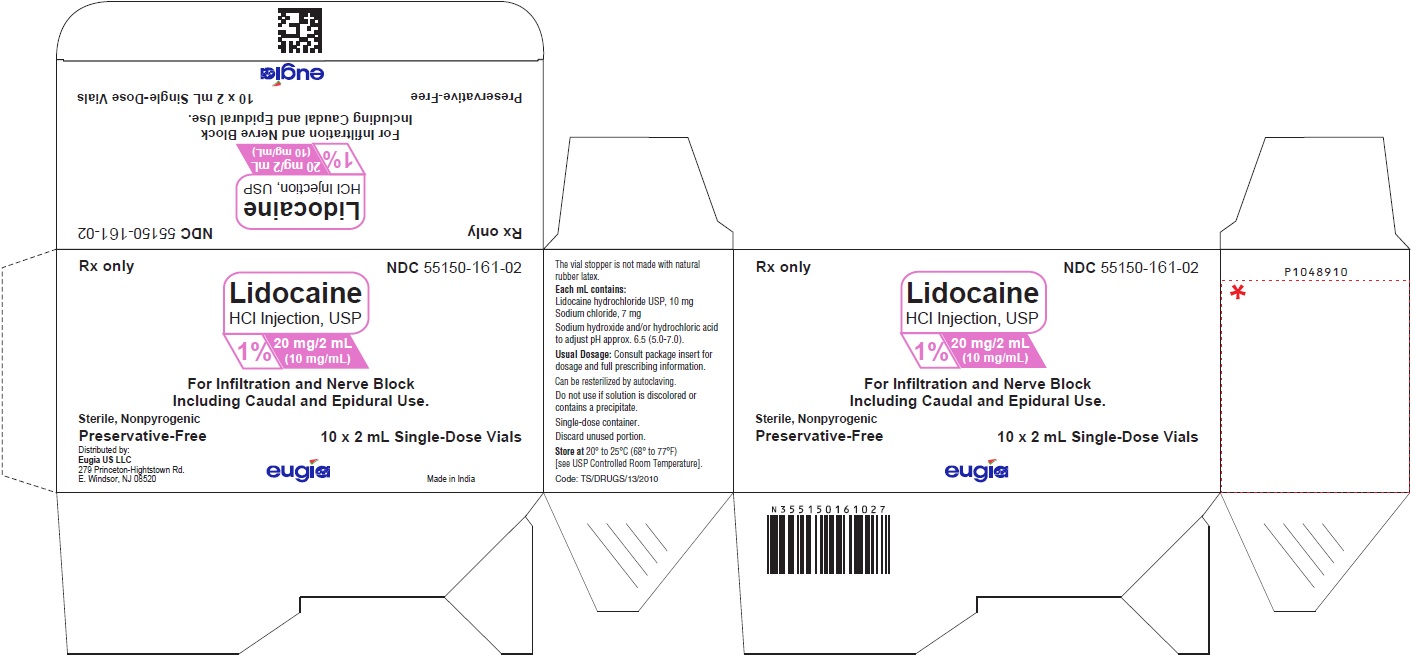
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1% 20 mg/2 mL (10 mg/mL) - 2 mL Container LabelRx only NDC 55150-161-02 - Lidocaine - HCl Injection, USP - 1% 20 mg/2 mL - (10 mg/mL) Preservative-Free - 2 mL Single-Dose Vial
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1% 20 mg/2 mL (10 mg/mL) - 2 mL Container-Carton [10 Vials]Rx only NDC 55150-161-02 - Lidocaine - HCl Injection, USP - 1% 20 mg/2 mL - (10 mg/mL) For Infiltration and Nerve Block - Including Caudal and Epidural Use. Sterile ...
-
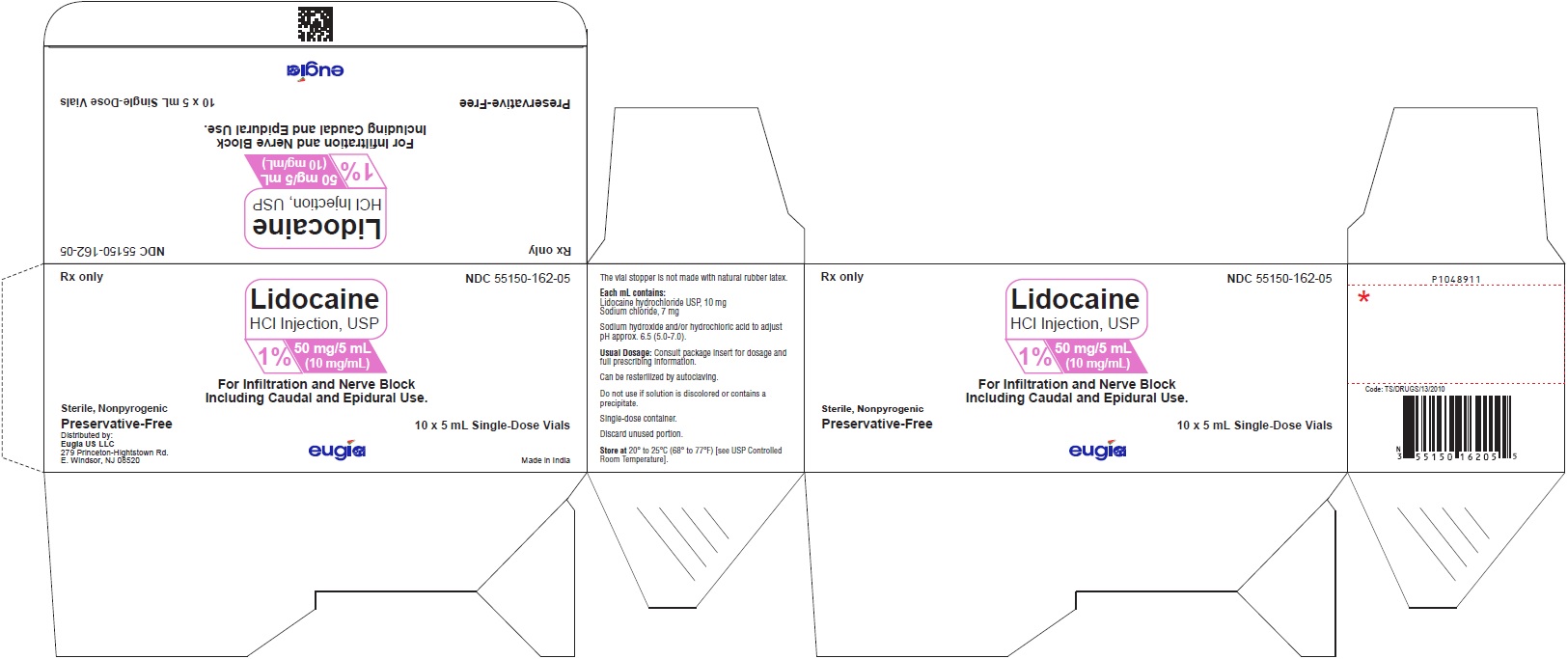
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1% 50 mg/5 mL (10 mg/mL) - 5 mL Container LabelRx only NDC 55150-162-05 - Lidocaine - HCl Injection, USP - 1% 50 mg/5 mL - (10 mg/mL) For Infiltration and Nerve Block - Including Caudal and Epidural ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1% 50 mg/5 mL (10 mg/mL) - 5 mL Container-Carton [10 Vials]Rx only NDC 55150-162-05 - Lidocaine - HCl Injection, USP - 1% 50 mg/5 mL - (10 mg/mL) For Infiltration and Nerve Block - Including Caudal and Epidural Use ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1% 300 mg/30 mL (10 mg/mL) - 30 mL Container LabelRx only NDC 55150-163-30 - Lidocaine - HCl Injection, USP - 1% 300 mg/30 mL - (10 mg/mL) For Infiltration and Nerve Block - Including Caudal and Epidural ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1% 300 mg/30 mL (10 mg/mL) - 30 mL Container-Carton [1 Vial]Rx only NDC 55150-163-30 - Lidocaine - HCl Injection, USP - 1% 300 mg/30 mL - (10 mg/mL) For Infiltration and Nerve Block - Including Caudal and Epidural ...
-
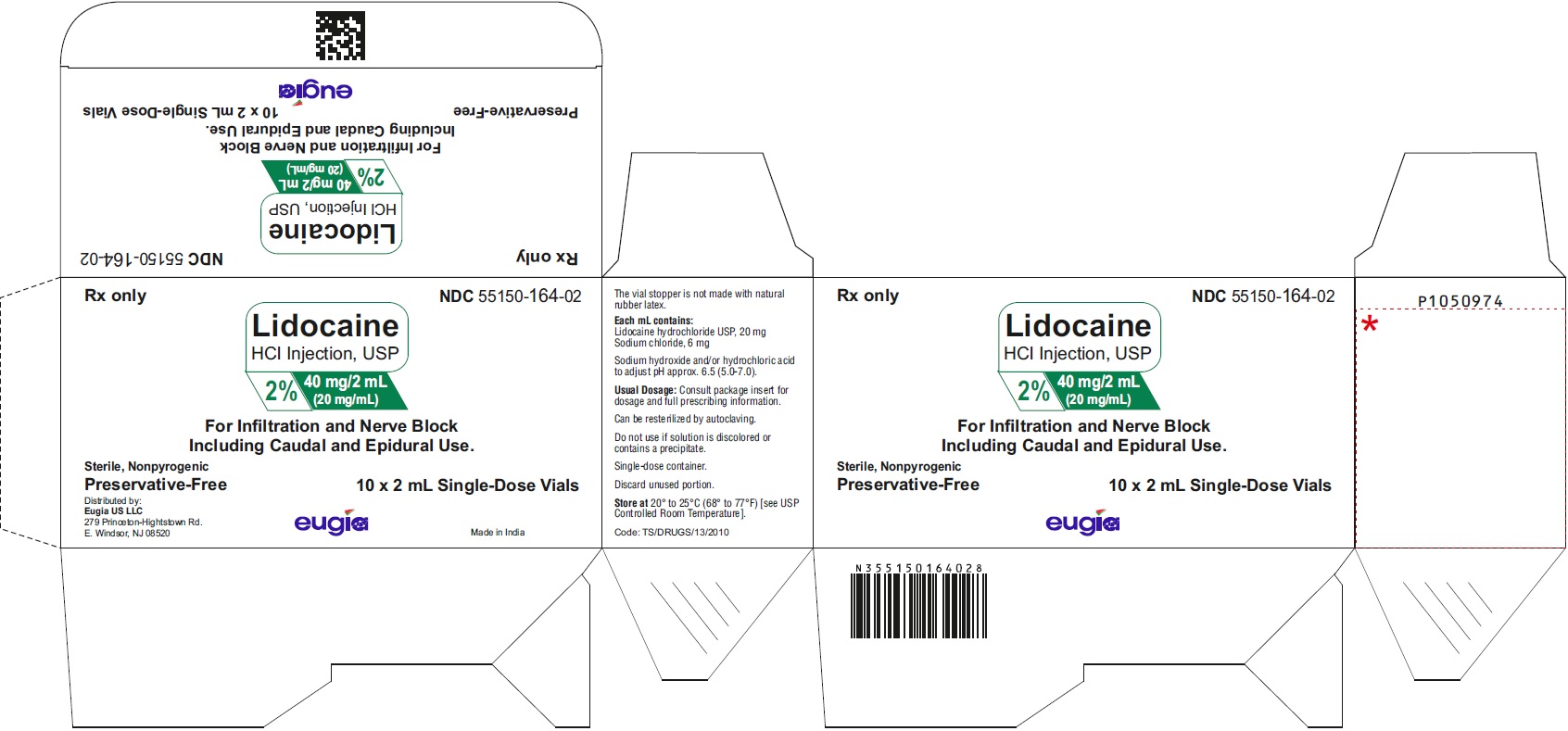
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 2% 40 mg/2 mL (20 mg/mL) - 2 mL Container LabelRx only NDC 55150-164-02 - Lidocaine - HCl Injection, USP - 2% 40 mg/2 mL - (20 mg/mL) Preservative-Free - 2 mL Single-Dose Vial
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 2% 40 mg/2 mL (20 mg/mL) - 2 mL Container-Carton [10 Vials]Rx only NDC 55150-164-02 - Lidocaine - HCl Injection, USP - 2% 40 mg/2 mL - (20 mg/mL) For Infiltration and Nerve Block - Including Caudal and Epidural ...
-
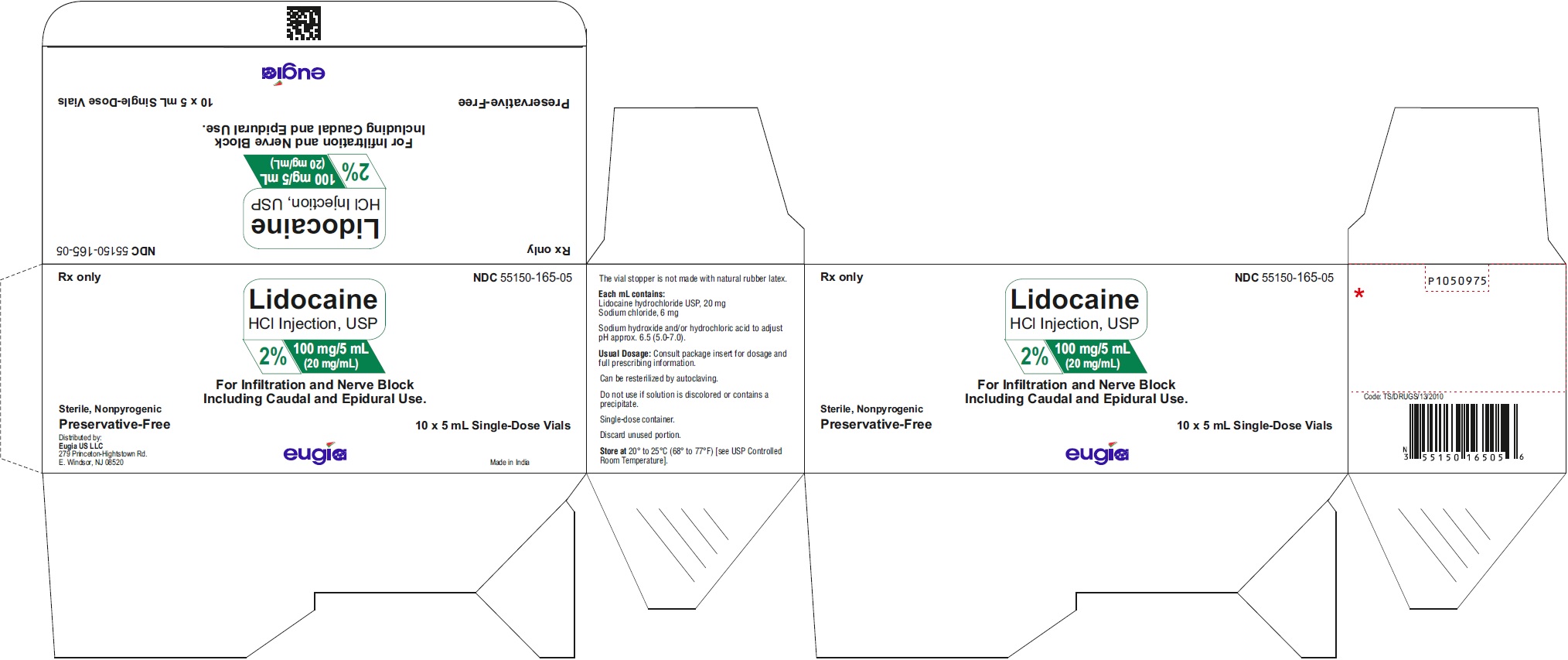
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 2% 100 mg/5 mL (20 mg/mL) - 5 mL Container LabelRx only NDC 55150-165-05 - Lidocaine - HCl Injection, USP - 2% 100 mg/5 mL - (20 mg/mL) For Infiltration and Nerve Block - Including Caudal and Epidural ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 2% 100 mg/5 mL (20 mg/mL) - 5 mL Container-Carton [10 Vials]Rx only NDC 55150-165-05 - Lidocaine - HCl Injection, USP - 2% 100 mg/5 mL - (20 mg/mL) For Infiltration and Nerve Block - Including Caudal and Epidural Use ...
-
INGREDIENTS AND APPEARANCEProduct Information







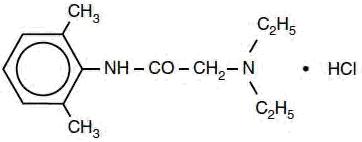

![PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1% 20 mg/2 mL (10 mg/mL) - 2 mL Container-Carton [10 Vials]](/dailymed/image.cfm?name=lidocaine-fig2.jpg&setid=89701fac-7536-4f16-87fb-f4cc121e74ee)

![PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1% 50 mg/5 mL (10 mg/mL) - 5 mL Container-Carton [10 Vials]](/dailymed/image.cfm?name=lidocaine-fig4.jpg&setid=89701fac-7536-4f16-87fb-f4cc121e74ee)

![PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1% 300 mg/30 mL (10 mg/mL) - 30 mL Container-Carton [1 Vial]](/dailymed/image.cfm?name=lidocaine-fig6.jpg&setid=89701fac-7536-4f16-87fb-f4cc121e74ee)

![PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 2% 40 mg/2 mL (20 mg/mL) - 2 mL Container-Carton [10 Vials]](/dailymed/image.cfm?name=lidocaine-fig8.jpg&setid=89701fac-7536-4f16-87fb-f4cc121e74ee)

![PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 2% 100 mg/5 mL (20 mg/mL) - 5 mL Container-Carton [10 Vials]](/dailymed/image.cfm?name=lidocaine-fig10.jpg&setid=89701fac-7536-4f16-87fb-f4cc121e74ee)