Label: IRBESARTAN AND HYDROCHLOROTHIAZIDE tablet, film coated
- NDC Code(s): 71335-1392-1, 71335-1392-2, 71335-1392-3
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 0955-1045
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated May 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Irbesartan and Hydrochlorothiazide safely and effectively. See full prescribing information for Irbesartan and Hydrochlorothiazide ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: FETAL TOXICITY
- When pregnancy is detected, discontinue irbesartan and hydrochlorothiazide as soon as possible [see Warnings and Precautions (5.1)and Use in Specific Populations (8.1)].
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus [see Warnings and Precautions (5.1)and Use in Specific Populations (8.1)].
-
1 INDICATIONS AND USAGEIrbesartan and hydrochlorothiazide tablets are indicated for the treatment of hypertension. Irbesartan and hydrochlorothiazide may be used in patients whose blood pressure is not adequately ...
-
2 DOSAGE AND ADMINISTRATION2.1 General Considerations - The side effects of irbesartan are generally rare and apparently independent of dose; those of hydrochlorothiazide are a mixture of dose-dependent (primarily ...
-
3 DOSAGE FORMS AND STRENGTHSIrbesartan and hydrochlorothiazide 150/12.5 mg and 300/12.5 mg film-coated tablets are peach, biconvex, and oval with a heart debossed on one side and "2875" or "2876" on the reverse side ...
-
4 CONTRAINDICATIONSIrbesartan and hydrochlorothiazide is contraindicated in patients who are hypersensitive to any component of this product. Because of the hydrochlorothiazide component, this product is ...
-
5 WARNINGS AND PRECAUTIONS5.1 Fetal Toxicity - Irbesartan and hydrochlorothiazide can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Nonsteroidal Anti-inflammatory Agents Including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors) Irbesartan - In patients who are elderly, volume depleted (including those on ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Irbesartan and hydrochlorothiazide can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the ...
-
10 OVERDOSAGEIrbesartan - No data are available in regard to overdosage in humans. However, daily doses of 900 mg for 8 weeks were well tolerated. The most likely manifestations of overdosage are expected ...
-
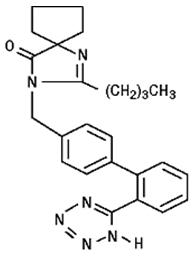
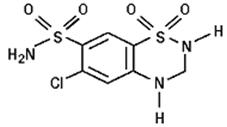
11 DESCRIPTIONIrbesartan and hydrochlorothiazide tablets are a combination of an angiotensin II receptor antagonist (AT - 1subtype), irbesartan, and a thiazide diuretic, hydrochlorothiazide (HCTZ) ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Irbesartan - Angiotensin II is a potent vasoconstrictor formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II) ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Irbesartan and Hydrochlorothiazide - No carcinogenicity studies have been conducted with the irbesartan and hydrochlorothiazide ...
-
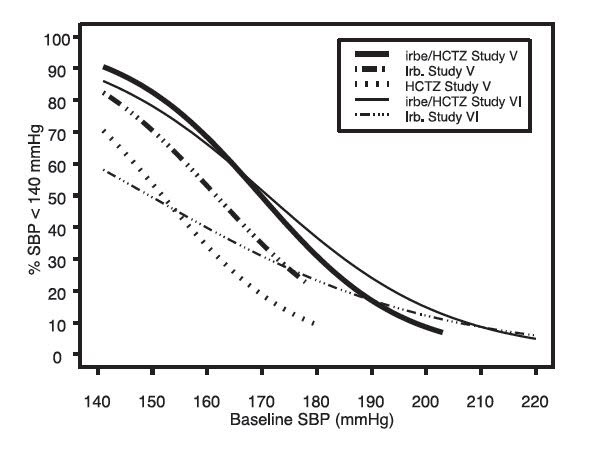
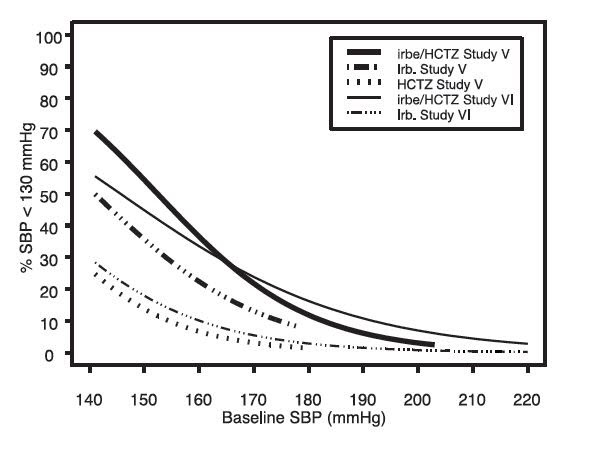
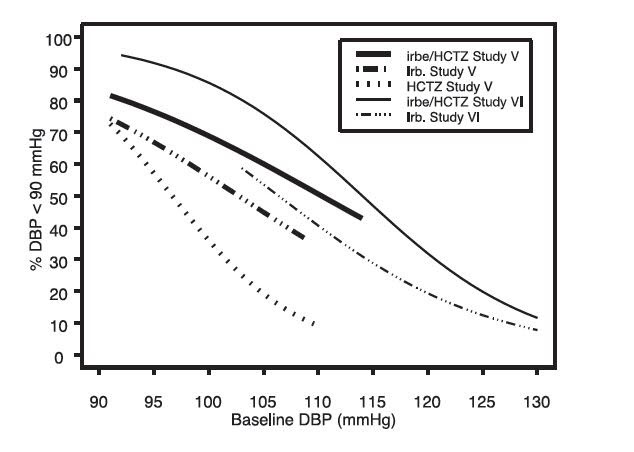
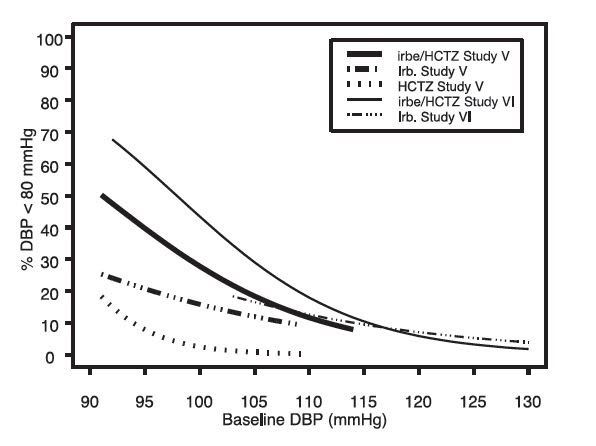
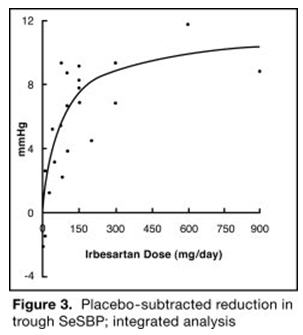
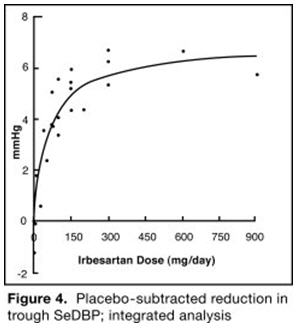
14 CLINICAL STUDIES14.1 Irbesartan Monotherapy - The antihypertensive effects of irbesartan were examined in 7 major placebo-controlled, 8 to 12-week trials in patients with baseline diastolic blood pressures of 95 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Irbesartan and hydrochlorothiazide film-coated tablets have markings on both sides and are available in the strengths and packages listed in the following table: (peach ...
-
17 PATIENT COUNSELING INFORMATIONPregnancy - Tell female patients of childbearing age about the consequences of exposure to irbesartan and hydrochlorothiazide during pregnancy. Discuss treatment options with women planning to ...
-
SPL UNCLASSIFIED SECTIONWinthrop U.S., a business of sanofi-aventis U.S. LLC - Bridgewater, NJ 08807 - A SANOFI COMPANY - ©2021 sanofi-aventis U.S. LLC
-
PRINCIPAL DISPLAY PANELIrbesartan and Hydrochlorothiazide Tablets 150 mg/12.5 mg
-
INGREDIENTS AND APPEARANCEProduct Information