Label: VAQTA- hepatitis a vaccine, inactivated injection, suspension
-
NDC Code(s):
0006-4095-01,
0006-4095-02,
0006-4096-01,
0006-4096-02, view more0006-4831-01, 0006-4831-41, 0006-4841-00, 0006-4841-01, 0006-4841-41
- Packager: Merck Sharp & Dohme LLC
- Category: VACCINE LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated November 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VAQTA safely and effectively. See full prescribing information for VAQTA.
VAQTA® (Hepatitis A Vaccine, Inactivated)
Suspension for Intramuscular Injection
Initial U.S. Approval: 1996INDICATIONS AND USAGE
VAQTA is a vaccine indicated for the prevention of disease caused by hepatitis A virus (HAV) in persons 12 months of age and older. The primary dose should be given at least 2 weeks prior to expected exposure to HAV. (1.1)
DOSAGE AND ADMINISTRATION
- For intramuscular administration only. (2)
- Children/Adolescents: vaccination consists of a 0.5-mL primary dose administered intramuscularly, and a 0.5-mL booster dose administered intramuscularly 6 to 18 months later. (2.1)
- Adults: vaccination consists of a 1-mL primary dose administered intramuscularly, and a 1-mL booster dose administered intramuscularly 6 to 18 months later. (2.1)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- The vial stopper and the syringe plunger stopper and tip cap contain dry natural latex rubber that may cause allergic reactions in latex-sensitive individuals. (5.2)
ADVERSE REACTIONS
The most common local adverse reactions and systemic adverse events (≥15%) reported in different clinical trials across different age groups when VAQTA was administered alone or concomitantly were:
- Children — 12 through 23 months of age: injection-site pain/tenderness (37.0%), injection-site erythema (21.2%), fever (16.4% when administered alone, and 27.0% when administered concomitantly) (6.1)
- Children/Adolescents — 2 through 18 years of age: injection-site pain (18.7%) (6.1)
- Adults — 19 years of age and older: injection-site pain, tenderness, or soreness (67.0%), injection-site warmth (18.2%) and headache (16.1%) (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme LLC at 1-877-888-4231 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov .
DRUG INTERACTIONS
- Do not mix VAQTA with any other vaccine in the same syringe or vial. (7.1)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Indications and Use
2 DOSAGE AND ADMINISTRATION
2.1 Dosage and Schedule
2.2 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Prevention and Management of Allergic Vaccine Reactions
5.2 Hypersensitivity to Latex
5.3 Altered Immunocompetence
5.4 Limitations of Vaccine Effectiveness
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
7.1 Use with Other Vaccines
7.2 Use with Immune Globulin
7.3 Immunosuppressive Therapy
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Immunocompromised Individuals
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Efficacy of VAQTA: The Monroe Clinical Study
14.2 Other Clinical Studies
14.3 Timing of Booster Dose Administration
14.4 Duration of Immune Response
14.5 Concomitant Administration of VAQTA and Immune Globulin
14.6 Interchangeability of the Booster Dose
14.7 Immune Response to Concomitantly Administered Vaccines
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
FOR INTRAMUSCULAR ADMINISTRATION ONLY.
2.1 Dosage and Schedule
Children/Adolescents (12 months through 18 years of age): The vaccination schedule consists of a primary 0.5-mL dose administered intramuscularly, and a 0.5-mL booster dose administered intramuscularly 6 to 18 months later.
Adults (19 years of age and older): The vaccination schedule consists of a primary 1-mL dose administered intramuscularly, and a 1-mL booster dose administered intramuscularly 6 to 18 months later.
Booster Immunization Following Another Manufacturer's Hepatitis A Vaccine: A booster dose of VAQTA may be given at 6 to 12 months following a primary dose of HAVRIX [see Clinical Studies (14.6)].
2.2 Preparation and Administration
Shake the single-dose vial or single-dose prefilled syringe well to obtain a slightly opaque, white suspension before withdrawal and use. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Discard if the suspension does not appear homogenous or if extraneous particulate matter remains or discoloration is observed.
For single-dose vials, withdraw and administer entire dose of VAQTA intramuscularly using a sterile needle and syringe. Discard vial after use.
For single-dose prefilled syringes, securely attach a needle by twisting in a clockwise direction and administer dose of VAQTA intramuscularly. Discard syringe after use.
For adults, adolescents, and children older than 2 years of age, the deltoid muscle is the preferred site for intramuscular injection. For children 12 through 23 months of age, the anterolateral area of the thigh is the preferred site for intramuscular injection.
-
3 DOSAGE FORMS AND STRENGTHS
Suspension for injection available in four presentations:
- 0.5-mL pediatric dose in single-dose vials and prefilled syringes
- 1-mL adult dose in single-dose vials and prefilled syringes
[See Description (11) for listing of vaccine components and How Supplied/Storage and Handling (16).]
-
4 CONTRAINDICATIONS
Do not administer VAQTA to individuals with a history of immediate and/or severe allergic or hypersensitivity reactions (e.g., anaphylaxis) after a previous dose of any hepatitis A vaccine, or to individuals who have had an anaphylactic reaction to any component of VAQTA, including neomycin [see Description (11)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Prevention and Management of Allergic Vaccine Reactions
Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of the vaccine [see Contraindications (4)].
5.2 Hypersensitivity to Latex
The vial stopper and the syringe plunger stopper and tip cap contain dry natural latex rubber that may cause allergic reactions in latex-sensitive individuals [see How Supplied/Storage and Handling (16)].
5.3 Altered Immunocompetence
Immunocompromised persons, including individuals receiving immunosuppressive therapy, may have a diminished immune response to VAQTA and may not be protected against HAV infection after vaccination [see Use in Specific Populations (8.6)].
5.4 Limitations of Vaccine Effectiveness
Hepatitis A virus has a relatively long incubation period (approximately 20 to 50 days). VAQTA may not prevent hepatitis A infection in individuals who have an unrecognized hepatitis A infection at the time of vaccination. Vaccination with VAQTA may not result in a protective response in all susceptible vaccinees.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
The safety of VAQTA has been evaluated in over 10,000 subjects 1 year to 85 years of age. Subjects were given one or two doses of the vaccine. The second (booster dose) was given 6 months or more after the first dose.
The most common local adverse reactions and systemic adverse events (≥15%) reported in different clinical trials across different age groups when VAQTA was administered alone or concomitantly were:
- Children — 12 through 23 months of age: injection-site pain/tenderness (37.0%), injection-site erythema (21.2%), fever (16.4% when administered alone, and 27.0% when administered concomitantly).
- Children/Adolescents — 2 through 18 years of age: injection-site pain (18.7%)
- Adults — 19 years of age and older: injection-site pain, tenderness, or soreness (67.0%), injection-site warmth (18.2%) and headache (16.1%)
Allergic Reactions
Local and/or systemic allergic reactions that occurred in <1% of over 10,000 children/adolescents or adults in clinical trials regardless of causality included: injection-site pruritus and/or rash; bronchial constriction; asthma; wheezing; edema/swelling; rash; generalized erythema; urticaria; pruritus; eye irritation/itching; dermatitis [see Contraindications (4) and Warnings and Precautions (5.1)].
Children — 12 through 23 Months of Age
Across five clinical trials, 4374 children 12 to 23 months of age received one or two 25U doses of VAQTA, including 3885 children who received 2 doses of VAQTA and 1250 children who received VAQTA concomitantly with one or more other vaccines, including Measles, Mumps, and Rubella Virus Vaccine, Live (M-M-R II®), Varicella Vaccine, Live (VARIVAX®), Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine, Adsorbed (Tripedia or INFANRIX), Measles, Mumps, Rubella, and Varicella Vaccine, Live (ProQuad®), Pneumococcal 7-valent Conjugate Vaccine (Diphtheria CRM197, Prevnar), or Haemophilus B Conjugate Vaccine (Meningococcal Protein Conjugate, PedvaxHIB®). Overall, the race distribution of study subjects was as follows: 64.7% Caucasian; 15.7% Hispanic-American; 12.3% Black; 4.8% other; 1.4% Asian; and 1.1% Native American. The distribution of subjects by gender was 51.8% male and 48.2% female.
In an open-label clinical trial, 653 children 12 to 23 months of age were randomized to receive a first dose of VAQTA with ProQuad and Prevnar concomitantly (N=330) or a first dose of ProQuad and pneumococcal 7-valent conjugate vaccine concomitantly, followed by a first dose of VAQTA 6 weeks later (N=323). Approximately 6 months later, subjects received either the second doses of ProQuad and VAQTA concomitantly or the second doses of ProQuad and VAQTA separately. The race distribution of the study subjects was as follows: 60.3% Caucasian; 21.6% African-American; 9.5% Hispanic-American; 7.2% other; 1.1% Asian; and 0.3% Native American. The distribution of subjects by gender was 50.7% male and 49.3% female.
Table 1 presents rates of solicited local reactions at the VAQTA injection site and rates of elevated temperatures (≥100.4°F and ≥102.2°F) that occurred within 5 days following each dose of VAQTA and elevated temperatures >98.6°F for a total of 14 days after vaccination; occurrences of these events were recorded daily on diary cards. Table 2 presents rates of unsolicited systemic adverse events that occurred within 14 days at ≥5% in any group following each dose of VAQTA.
Table 1: Incidences of Solicited Local Adverse Reactions at the VAQTA Injection Site and Elevated Temperatures Following Each Dose of VAQTA in Healthy Children 12-23 Months of Age Receiving VAQTA Alone or Concomitantly With ProQuad and PREVNAR* Dose 1 Dose 2 Adverse reaction: Days 1-5 unless noted VAQTA alone VAQTA + ProQuad + Prevnar concomitantly VAQTA alone VAQTA + ProQuad concomitantly N=number of subjects for whom data are available. - *
- Pneumococcal 7-valent Conjugate Vaccine
Injection site adverse reactions N=274 N=311 N=251 N=263 Injection site erythema 11.7% 9.6% 12.7% 9.5% Injection site pain/tenderness 15.3% 20.9% 20.3% 17.5% Injection site swelling 9.5% 6.8% 7.6% 6.1% Temperature > 98.6°F or feverish (Days 1-14) 12.4% 35.7% 10.8% 10.3% N=243 N=285 N=221 N=237 Temperature ≥ 100.4°F 10.3% 16.8% 10% 4.2% Temperature ≥ 102.2 °F 2.1% 3.5% 2.3% 2.5% Table 2: Incidences of Unsolicited Systemic Adverse Events ≥5% in Any Group Following Each Dose of VAQTA in Healthy Children 12-23 Months of Age Receiving VAQTA Alone or Concomitantly With ProQuad and PREVNAR* Dose 1 Dose 2 Adverse Event: Days 1-14 VAQTA alone VAQTA + ProQuad + PREVNAR concomitantly VAQTA alone VAQTA + ProQuad concomitantly - *
- Pneumococcal 7-valent Conjugate Vaccine
N=274 N=311 N=251 N=263 General Disorders and Administration Site Conditions Irritability 3.6% 6.1% 2.8% 2.7% Infections and Infestations Upper respiratory tract infection 3.3% 6.1% 4.8% 5.7% Skin and Subcutaneous Tissue Disorders Dermatitis diaper 1.1% 6.1% 2.4% 3.4% In Stage I of an open, multicenter, randomized study, children 15 months of age were randomized to receive the first dose of VAQTA alone (N=151) or concomitantly with PedvaxHIB and INFANRIX (N=155); another group of children 15 months of age were randomized to receive the first dose of VAQTA alone (N=152) or concomitantly with PedvaxHIB (N=159). All groups received the second dose of VAQTA alone at least 6 months following the first dose. The race distribution of Stage I study subjects was: 63.9% Caucasian; 17.5% Hispanic-American; 14.7% Black; 2.6% other; and 1.3% Asian. The distribution of subjects by gender was 54.0% male and 46.0% female. In Stage II of this study, an additional 654 children 12-17 months of age received the first dose of VAQTA alone followed by the second dose of VAQTA 6 months later. The race distribution of Stage II of the study subjects was: 66.1% Caucasian; 10.6% Hispanic-American; 16.8% Black; 4.7% other; and 1.5% Asian. The distribution of subjects by gender was 51.2% male and 48.8% female.
Table 3 presents rates of solicited local reactions at the VAQTA injection-site and rates of elevated temperatures (≥100.4°F and ≥102.2°F) that occurred within 5 days following each dose of VAQTA and elevated temperatures >98.6°F for a total of 14 days following each dose of VAQTA. Occurrences of these events were recorded daily on diary cards. Table 4 presents rates of unsolicited systemic adverse events that occurred within 14 days at ≥5% following each dose of VAQTA.
Table 3: Incidences of Solicited Local Adverse Reactions at the VAQTA Injection Site and Elevated Temperatures Following Each Dose of VAQTA in Healthy Children 12-23 Months of Age Receiving VAQTA Alone or Concomitantly with PedvaxHIB With or Without INFANRIX (Stage I) and those Receiving VAQTA Alone at Both Doses (Stage II) Stage I Stage II Dose 1 Dose 2 Dose 1 Dose 2 Adverse Reaction: Days 1-5 unless noted VAQTA alone VAQTA + PedvaxHIB and Infanrix or VAQTA + PedvaxHIB concomitantly VAQTA alone VAQTA alone VAQTA alone N= number of subjects for whom data is available Injection site adverse reactions N=256 N=302 N=503 N=647 N=599 Injection site erythema 18.0% 19.9% 21.5% 11.7% 16.2% Injection site pain/tenderness 21.9% 36.4% 27.4% 20.1% 22.9% Injection site swelling 10.2% 14.2% 10.1% 7.1% 7.0% Temperature > 98.6°F or feverish (Days 1-14) 10.2% 17.2% 10.7% 10.0% 8.2% N=234 N=290 N=473 N=631 N=591 Temperature ≥ 100.4°F 9.0% 16.9% 9.1% 9.4% 8.6% Temperature ≥ 102.2 °F 3.8% 3.1% 3.2% 2.9% 2.4% Table 4: Incidences of Unsolicited Systemic Adverse Events ≥5% in Any Group Following Each Dose of VAQTA in Healthy Children 12-23 Months of Age Receiving VAQTA Alone or Concomitantly with PedvaxHIB With or Without INFANRIX (Stage I) and Those Receiving VAQTA Alone at Both Doses (Stage II) Stage I Stage II Dose 1 Dose 2 Dose 1 Dose 2 Adverse Event:
Days 1-14VAQTA alone VAQTA + PedvaxHIB and Infanrix or
VAQTA + PedvaxHIB concomitantlyVAQTA alone VAQTA alone VAQTA alone N=256 N=302 N=503 N=647 N=599 Gastrointestinal Disorders Diarrhea 3.9% 8.3% 3.8% 4.6% 3.8% Teething 3.1% 2.3% 1.4% 5.7% 4.3% General Disorders and Administration Site Conditions Irritability 6.3% 9.6% 4.0% 8.8% 6.5% Infections and Infestations Upper respiratory tract infection 2.3% 3.3% 3.0% 4.9% 5.2% Respiratory, Thoracic and Mediastinal Disorders Rhinorrhea 2.0% 4.0% 3.8% 6.2% 3.8% Data presented in Tables 1 through 4 on solicited local reactions, and solicited and unsolicited systemic adverse events with incidence ≥5% following each dose of VAQTA are representative of other clinical trials of VAQTA in children 12 through 23 months of age. Across the five studies conducted in children 12-23 months of age, ≥39.9% of subjects experienced local adverse reactions and ≥55.7% of subjects experienced systemic adverse events. The majority of local and systemic adverse events were mild to moderate in intensity.
The following additional unsolicited local adverse reactions and systemic adverse events were observed at a common frequency of ≥1% to <10% in any individual clinical study. This listing includes only the adverse reactions not reported elsewhere in the label. These local adverse reactions and systemic adverse events occurred among recipients of VAQTA alone or VAQTA given concomitantly within 14 days following any dose of VAQTA across four clinical studies.
- Eye disorders: Conjunctivitis
- Gastrointestinal disorders: Constipation; vomiting
- General disorders and administration site conditions: Injection-site bruising; injection-site ecchymosis
- Infections and infestations: Otitis media; nasopharyngitis; rhinitis; viral infection; croup; pharyngitis streptococcal; laryngotracheobronchitis; viral exanthema; gastroenteritis viral; roseola
- Metabolism and nutrition disorders: Anorexia
- Psychiatric disorders: Insomnia; crying
- Respiratory, thoracic and mediastinal disorders: Cough; nasal congestion; respiratory congestion
- Skin and subcutaneous tissue disorders: Rash vesicular; measles-like/rubella-like rash; varicella-like rash; rash morbilliform
Serious Adverse Events (Children 12 through 23 Months of Age): Across the five studies conducted in subjects 12-23 months of age, 0.7% (32/4374) of subjects reported a serious adverse event following any dose of VAQTA, and 0.1% (5/4374) of subjects reported a serious adverse event judged to be vaccine related by the study investigator. The serious adverse events were collected over the period defined in each protocol (14, 28, or 42 days). Vaccine-related serious adverse events which occurred following any dose of VAQTA with or without concomitant vaccines included febrile seizure (0.05%), dehydration (0.02%), gastroenteritis (0.02%), and cellulitis (0.02%).
Children/Adolescents — 2 Years through 18 Years of Age
In 11 clinical trials, 2615 healthy children 2 years through 18 years of age received at least one dose of VAQTA. These studies included administration of VAQTA in varying doses and regimens (1377 children received one or more 25U doses). The race distribution of the study subjects who received at least one dose of VAQTA in these studies was as follows: 84.7% Caucasian; 10.6% American Indian; 2.3% African-American; 1.5% Hispanic-American; 0.6% other; 0.2% Oriental. The distribution of subjects by gender was 51.2% male and 48.8% female.
In a double-blind, placebo-controlled efficacy trial (i.e. The Monroe Efficacy Study), 1037 healthy children and adolescents 2 through 16 years of age were randomized to receive a primary dose of 25U of VAQTA and a booster dose of VAQTA 6, 12, or 18 months later, or placebo (alum diluent). All study subjects were Caucasian: 51.5% were male and 48.5% were female. Subjects were followed days 1 to 5 postvaccination for fever and local adverse reactions and days 1 to 14 for systemic adverse events. The most common adverse events/reactions were injection-site reactions, reported by 6.4% of subjects. Table 5 summarizes local adverse reactions and systemic adverse events reported in ≥1% of subjects. There were no significant differences in the rates of any adverse events or adverse reactions between vaccine and placebo recipients after Dose 1.
Table 5: Local Adverse Reactions and Systemic Adverse Events (≥1%) in Healthy Children and Adolescents from the Monroe Efficacy Study Adverse Event VAQTA
(N=519)Placebo (Alum Diluent)*,†,‡
(N=518)
Rate (Percent)Dose 1*
Rate (Percent)Booster
Rate (Percent)N=Number of subjects enrolled/randomized. Percent=percentage of subjects for whom data are available with adverse event n=number of subjects for whom adverse events available - *
- No statistically significant differences between the two groups.
- †
- Second injection of placebo not administered because code for the trial was broken.
- ‡
- Placebo (Alum diluent) = amorphous aluminum hydroxyphosphate sulfate.
- §
- Adverse Reactions at the injection site (VAQTA) Days 1-5 after vaccination with VAQTA
- ¶
- Systemic adverse events reported Days 1-15 after vaccination, regardless of causality.
Injection Site§ n=515 n=475 n=510 Pain 6.4% 3.4% 6.3% Tenderness 4.9% 1.7% 6.1% Erythema 1.9% 0.8% 1.8% Swelling 1.7% 1.5% 1.6% Warmth 1.7% 0.6% 1.6% Systemic¶ n=519 n=475 n=518 Abdominal pain 1.2% 1.1% 1.0% Pharyngitis 1.2% 0% 0.8% Headache 0.4% 0.8% 1.0% Adults — 19 Years of Age and Older
In an open-label clinical trial, 240 healthy adults 18 to 54 years of age were randomized to receive either VAQTA (50U/1-mL) with Typhim Vi (Typhoid Vi polysaccharide vaccine) and YF-Vax (yellow fever vaccine) concomitantly (N=80), typhoid Vi polysaccharide and yellow fever vaccines concomitantly (N=80), or VAQTA alone (N=80). Approximately 6 months later, subjects who received VAQTA were administered a second dose of VAQTA. The race distribution of the study subjects who received VAQTA with or without typhoid Vi polysaccharide and yellow fever vaccine was as follows: 78.3% Caucasian; 14.2% Oriental; 3.3% other; 2.1% African-American; 1.7% Indian; 0.4% Hispanic-American. The distribution of subjects by gender was 40.8% male and 59.2% female. Subjects were monitored for local adverse reactions and fever for 5 days and systemic adverse events for 14 days after each vaccination. In the 14 days after the first dose of VAQTA, the proportion of subjects with adverse events was similar between recipients of VAQTA given concomitantly with typhoid Vi polysaccharide and yellow fever vaccines compared to recipients of typhoid Vi polysaccharide and yellow fever vaccines without VAQTA. Table 6 summarizes solicited local adverse reactions and Table 7 summarizes unsolicited systemic adverse events reported in ≥5% in adults who received one or two doses of VAQTA alone and for subjects who received VAQTA concomitantly with typhoid Vi polysaccharide and yellow fever vaccines. There were no solicited systemic complaints reported at a rate ≥5%. Fever ≥101°F occurred in 1.3% of subjects in each group.
Table 6: Incidences of Solicited Local Adverse Reactions in Healthy Adults ≥19 Years of Age Occurring at ≥5% After Any Dose Adverse Event VAQTA administered alone
(N=80)VAQTA + ViCPS* and Yellow Fever vaccines administered concomitantly†
(N=80)Rate (Percent) N=Number of subjects enrolled/randomized. Percent=percentage of subjects with adverse event. Injection-site‡ Pain/tenderness/soreness 78.8% 70.3% Warmth 23.7% 23.7% Swelling 16.2% 8.8% Erythema 17.5% 6.3% Table 7: Incidences of Unsolicited Systemic Adverse Events in Adults ≥19 Years of Age Occurring at ≥5% After Any Dose Body System VAQTA administered alone
(N=80)VAQTA + ViCPS* and Yellow Fever vaccines administered concomitantly†
(N=80)Adverse Event Rate (Percent) N=Number of subjects enrolled/randomized with data available. Percent=percentage of subjects with adverse event for whom data are available. General disorders and administration site reactions‡ Asthenia/fatigue 7.5% 11.3% Chills 1.3% 7.5% Gastrointestinal disorders‡ Nausea 7.5% 12.5% Musculoskeletal and connective tissue disorders‡ Myalgia 5.0% 10.0% Arm pain 0.0% 6.3% Nervous system disorders‡ Headache 23.8% 26.3% Infections and infestations‡ Upper respiratory infection 7.5% 3.8% Pharyngitis 2.5% 6.3% In four clinical trials involving 1645 healthy adults 19 years of age and older who received one or more 50U doses of hepatitis A vaccine, subjects were followed for fever and local adverse reactions 1 to 5 days postvaccination and for systemic adverse events 1 to 14 days postvaccination. One single-blind study evaluated doses of VAQTA with varying amounts of viral antigen and/or alum content in healthy adults ≥170 pounds and ≥30 years of age (N=210 adults administered 50U/1-mL dose). One open-label study evaluated VAQTA given with immune globulin (IG) or alone (N=164 adults who received VAQTA alone). A third study was single-blind and evaluated 3 different lots of VAQTA (N=1112). The fourth study that was also single-blind evaluated doses of VAQTA with varying amounts of viral antigen in healthy adults ≥170 pounds and ≥30 years of age (N=159 adults administered the 50U/1-mL dose). Overall, the race distribution of the study subjects who received at least one dose of VAQTA was as follows: 94.2% Caucasian; 2.2% Black; 1.5% Hispanic; 1.5% Oriental; 0.4% other; 0.2% American Indian. 47.6% of subjects were male and 52.4% were female. The most common adverse event/reaction was injection-site pain/soreness/tenderness reported by 67.0% of subjects. Of all reported injection-site reactions 99.8% were mild (i.e., easily tolerated with no medical intervention) or moderate (i.e., minimally interfered with usual activity possibly requiring little medical intervention). Listed below in Table 8 are the local adverse reactions and systemic adverse events reported by ≥5% of subjects, in decreasing order of frequency within each body system.
Table 8: Incidences of Local Adverse Reactions and Systemic Adverse Events ≥5% in Adults 19 Years of Age and Older Body System VAQTA (Any Dose)
(N=1645)Adverse Events Rate (n/total n) N=Number of subjects enrolled/randomized. n=Number of subjects in each category with data available. Percent=percentage of subjects for whom data are available with adverse event. Nervous system disorders* n=1641 Headache 16.1% General disorders and administration site reactions† n=1640 Injection-site pain/tenderness/soreness 67.0% Injection-site warmth 18.2% Injection-site swelling 14.7% Injection-site erythema 13.7% The following additional unsolicited systemic adverse events were observed among recipients of VAQTA that occurred within 14 days at a common frequency of ≥1% to <10% following any dose not reported elsewhere in the label. These adverse reactions have been reported across 4 clinical studies.
- Musculoskeletal and connective tissue disorders: Back pain; stiffness
- Reproductive system and breast disorders: Menstruation disorders
6.2 Post-Marketing Experience
The following additional adverse events have been reported with use of the marketed vaccine. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to a vaccine exposure.
Blood and lymphatic disorders: Thrombocytopenia.
Nervous system disorders: Guillain-Barré syndrome; cerebellar ataxia; encephalitis.Post-Marketing Observational Safety Study
In a post-marketing, 60-day safety surveillance study, conducted at a large health maintenance organization in the United States, a total of 42,110 individuals ≥2 years of age received 1 or 2 doses of VAQTA (13,735 children/adolescents and 28,375 adult subjects). Safety was passively monitored by electronic search of the automated medical records database for emergency room and outpatient visits, hospitalizations, and deaths. Medical charts were reviewed when an event was considered to be possibly vaccine-related by the investigator. None of the serious adverse events identified were assessed as being related to vaccine by the investigator. Diarrhea/gastroenteritis, resulting in outpatient visits, was determined by the investigator to be the only vaccine-related nonserious adverse reaction in the study. There was no vaccine-related adverse reaction identified that had not been reported in earlier clinical trials with VAQTA.
-
7 DRUG INTERACTIONS
7.1 Use with Other Vaccines
Do not mix VAQTA with any other vaccine in the same syringe or vial. Use separate injection sites and syringes for each vaccine. Please refer to package inserts of coadministered vaccines.
In clinical trials in children, VAQTA was concomitantly administered with one or more of the following US licensed vaccines: Measles, Mumps, and Rubella Virus Vaccine, Live; Varicella Vaccine, Live; Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine, Adsorbed; Measles, Mumps, Rubella, and Varicella Vaccine, Live; Pneumococcal 7-valent Conjugate Vaccine (Diphtheria CRM197); and Haemophilus B Conjugate Vaccine (Meningococcal Protein Conjugate). Safety and immunogenicity were similar for concomitantly administered vaccines compared to separately administered vaccines.
In clinical trials in adults, VAQTA was concomitantly administered with typhoid Vi polysaccharide and yellow fever vaccines [see Adverse Reactions (6.1) and Clinical Studies (14.2, 14.7)]. Safety and immunogenicity were similar for concomitantly administered vaccines compared to separately administered vaccines.
7.2 Use with Immune Globulin
VAQTA may be administered concomitantly with Immune Globulin, human, using separate sites and syringes. The recommended vaccination regimen for VAQTA should be followed. Consult the manufacturer's product circular for the appropriate dosage of Immune Globulin. A booster dose of VAQTA should be administered at the appropriate time as outlined in the recommended regimen for VAQTA [see Clinical Studies (14.5)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
There are no adequate and well-controlled studies designed to evaluate VAQTA in pregnant women. Available post-approval data do not suggest an increased risk of miscarriage or major birth defects in women who received VAQTA during pregnancy.
Developmental toxicity studies have not been conducted with VAQTA in animals.
Data
Human Data
Post-approval adverse reactions are reported voluntarily from a population of uncertain size. It is not always possible to reliably estimate their frequency or establish a causal relationship to the vaccine.
In prospectively reported spontaneous post-approval reports from 1995 to 2018, 36 women with a known pregnancy outcome were exposed to VAQTA during pregnancy following the last menstrual period. After excluding induced abortions (n=4) and those with exposure in the third trimester (n=2), there were 30 pregnancies with known outcomes with exposures in the first or second trimester. Miscarriage was reported for 3 of 30 (10%) pregnancies. Major birth defects were reported for 1 of 27 (3.7%) live born infants. The rates of miscarriage and major birth defects were consistent with estimated background rates.
8.2 Lactation
Risk Summary
It is not known whether VAQTA is excreted in human milk. Data are not available to assess the effects of VAQTA on the breastfed infant or on milk production/excretion.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for VAQTA and any potential adverse effects on the breastfed child from VAQTA or from the underlying maternal condition. For preventive vaccines the underlying condition is susceptibility to disease prevented by the vaccine.
8.4 Pediatric Use
The safety of VAQTA has been evaluated in 4374 children 12 through 23 months of age, and 2615 children/adolescents 2 through 18 years of age who received at least one 25U dose of VAQTA [see Adverse Reactions (6) and Dosage and Administration (2)].
Safety and effectiveness in infants below 12 months of age have not been established.
8.5 Geriatric Use
In the post-marketing observational safety study which included 42,110 persons who received VAQTA [see Adverse Reactions (6.2)], 4769 persons were 65 years of age or older and 1073 persons were 75 years of age or older. There were no adverse events judged by the investigator to be vaccine-related in the geriatric study population. In other clinical studies, 68 subjects 65 years of age or older were vaccinated with VAQTA, 10 of whom were 75 years of age or older. No overall differences in safety and immunogenicity were observed between these subjects and younger subjects; however, greater sensitivity of some older individuals cannot be ruled out. Other reported clinical experience has not identified differences in responses between the elderly and younger subjects.
-
11 DESCRIPTION
VAQTA is an inactivated whole virus vaccine derived from hepatitis A virus grown in cell culture in human MRC-5 diploid fibroblasts. It contains inactivated virus of a strain which was originally derived by further serial passage of a proven attenuated strain. The virus is grown, harvested, purified by a combination of physical and high performance liquid chromatographic techniques developed at the Research Laboratories of Merck Sharp & Dohme LLC, Rahway, NJ, USA, formalin inactivated, and then adsorbed onto amorphous aluminum hydroxyphosphate sulfate.
VAQTA is a sterile suspension for intramuscular injection. One milliliter of the vaccine contains approximately 50U of hepatitis A virus antigen, which is purified and formulated without a preservative. Within the limits of current assay variability, the 50U dose of VAQTA contains less than 0.1 mcg of non-viral protein, less than 4 × 10–6 mcg of DNA, less than 10–4 mcg of bovine albumin, and less than 0.8 mcg of formaldehyde. Other process chemical residuals are less than 10 parts per billion (ppb), including neomycin.
Each 0.5-mL pediatric dose contains 25U of hepatitis A virus antigen and adsorbed onto approximately 0.225 mg of aluminum provided as amorphous aluminum hydroxyphosphate sulfate, and 35 mcg of sodium borate as a pH stabilizer, in 0.9% sodium chloride.
Each 1-mL adult dose contains 50U of hepatitis A virus antigen and adsorbed onto approximately 0.45 mg of aluminum provided as amorphous aluminum hydroxyphosphate sulfate, and 70 mcg of sodium borate as a pH stabilizer, in 0.9% sodium chloride.
- 12 CLINICAL PHARMACOLOGY
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
VAQTA has not been evaluated for its carcinogenic or mutagenic potential, or its potential to impair fertility. [See Use in Specific Populations (8).]
-
14 CLINICAL STUDIES
14.1 Efficacy of VAQTA: The Monroe Clinical Study
The immunogenicity and protective efficacy of VAQTA were evaluated in a randomized, double-blind, placebo-controlled study involving 1037 susceptible healthy children and adolescents 2 through 16 years of age in a U.S. community with recurrent outbreaks of hepatitis A (The Monroe Efficacy Study). All of these children were Caucasian, and there were 51.5% male and 48.5% female. Each child received an intramuscular dose of VAQTA (25U) (N=519) or placebo (alum diluent) (N=518). Among those individuals who were initially seronegative (measured by a modification of the HAVAB radioimmunoassay [RIA]), seroconversion was achieved in >99% of vaccine recipients within 4 weeks after vaccination. The onset of seroconversion following a single dose of VAQTA was shown to parallel the onset of protection against clinical hepatitis A disease.
Because of the long incubation period of the disease (approximately 20 to 50 days, or longer in children), clinical efficacy was based on confirmed cases1 of hepatitis A occurring ≥50 days after vaccination in order to exclude any children incubating the infection before vaccination. In subjects who were initially seronegative, the protective efficacy of a single dose of VAQTA was observed to be 100% with 21 cases of clinically confirmed hepatitis A occurring in the placebo group and none in the vaccine group (p<0.001). The number of clinically confirmed cases of hepatitis A ≥30 days after vaccination were also compared. In this analysis, 28 cases of clinically confirmed hepatitis A occurred in the placebo group while none occurred in the vaccine group ≥30 days after vaccination. In addition, it was observed in this trial that no cases of clinically confirmed hepatitis A occurred in the vaccine group after day 16.2 Following demonstration of protection with a single dose and termination of the study, a booster dose was administered to a subset of vaccinees 6, 12, or 18 months after the primary dose.
No cases of clinically confirmed hepatitis A disease ≥50 days after vaccination have occurred in those vaccinees from The Monroe Efficacy Study monitored for up to 9 years.
- 1
- The clinical case definition included all of the following occurring at the same time: 1) one or more typical clinical signs or symptoms of hepatitis A (e.g., jaundice, malaise, fever ≥38.3°C); 2) elevation of hepatitis A IgM antibody (HAVAB-M); 3) elevation of alanine transferase (ALT) ≥2 times the upper limit of normal.
- 2
- One vaccinee did not meet the pre-defined criteria for clinically confirmed hepatitis A but did have positive hepatitis A IgM and borderline liver enzyme (ALT) elevations on days 34, 50, and 58 after vaccination with mild clinical symptoms observed on days 49 and 50.
14.2 Other Clinical Studies
The efficacy of VAQTA in other age groups was based upon immunogenicity measured 4 to 6 weeks following vaccination. VAQTA was found to be immunogenic in all age groups.
Children — 12 through 23 Months of Age
In a clinical trial, children 12 through 23 months of age were randomized to receive the first dose of VAQTA with or without M-M-R II and VARIVAX (N=617) and the second dose of VAQTA with or without Tripedia and optionally either oral poliovirus vaccine (no longer licensed in the US) or IPOL (N=555). The race distribution of study subjects who received at least one dose of VAQTA was as follows: 56.7% Caucasian; 17.5% Hispanic-American; 14.3% African-American; 7.0% Native American; 3.4% other; 0.8% Oriental; 0.2% Asian; and 0.2% Indian. The distribution of subjects by gender was 53.6% male and 46.4% female. In the analysis population, there were 471 initially seronegative children 12 through 23 months of age, who received the first dose of VAQTA with (N=237) or without (N=234) M-M-R II and VARIVAX of whom 96% (95% CI: 93.7%, 97.5%) seroconverted (defined as having an anti-HAV titer ≥10 mIU/mL) post dose 1 with an anti-HAV geometric mean titer (GMT) of 48 mIU/mL (95% CI: 44.7, 51.6). There were 343 children in the analysis population who received the second dose of VAQTA with (N=168) or without (N=175) Tripedia and optional oral poliovirus vaccine or IPOL of whom 100% (95% CI: 99.3%, 100%) seroconverted post dose 2 with an anti-HAV GMT of 6920 mIU/mL (95% CI: 6136, 7801). Of children who received only VAQTA at both visits, 100% (n=97) seroconverted after the second dose of VAQTA.
In a clinical trial involving 653 healthy children 12 to 15 months of age, 330 were randomized to receive VAQTA, ProQuad, and pneumococcal 7-valent conjugate vaccine concomitantly, and 323 were randomized to receive ProQuad and pneumococcal 7-valent conjugate vaccine concomitantly followed by VAQTA 6 weeks later. The race distribution of the study subjects was as follows: 60.3% Caucasian; 21.6% African-American; 9.5% Hispanic-American; 7.2% other; 1.1% Asian/Pacific; and 0.3% Native American. The distribution of subjects by gender was 50.7% male and 49.3% female. In the analysis population, the seropositivity rate for hepatitis A antibody (defined as the percent of subjects with an anti-HAV titer ≥10 mIU/mL) was 100% (n=182; 95% CI: 98.0%, 100%) post dose 2 with an anti-HAV GMT of 4977 mIU/mL (95% CI: 4068, 6089) when VAQTA was given with ProQuad and pneumococcal 7-valent conjugate vaccine and 99.4% (n=159, 95% CI: 96.5%, 100%) post dose 2 with an anti-HAV GMT of 6123 mIU/mL (95% CI: 4826, 7770) when VAQTA alone was given. These seropositivity rates were similar whether VAQTA was administered with or without ProQuad and pneumococcal 7-valent conjugate vaccine.
In an open, multicenter, randomized study involving 617 children 15 months of age, 306 were randomized to receive VAQTA with or without PedvaxHIB and INFANRIX, and 311 were randomized to receive VAQTA with or without PedvaxHIB. The race distribution of the study subjects was as follows: 63.9% Caucasian; 17.5% Hispanic-American; 14.7% Black; 2.6% other; and 1.3% Asian. The distribution of subjects by gender was 54.0% male and 46.0% female. The seropositivity rate for hepatitis A antibody (defined as the percent of subjects with an anti-HAV titer ≥ 10 mIU/mL) 4 weeks post dose 2 was 100% (n=208, 95% CI: 98.2%, 100.0%) in those who received VAQTA concomitantly with PedvaxHIB and INFANRIX or concomitantly with PedvaxHIB. In those subjects who received VAQTA alone, the seropositivity rate for hepatitis A antibody was 100% (n=183, 95% CI: 98.0%, 100.0%), regardless of baseline hepatitis A serostatus. Overall, the anti-HAV GMT in the concomitant groups was 3616.5 mIU/mL (95% CI: 3084.5, 4240.2). The anti-HAV GMT in the nonconcomitant groups was 4712.6 mIU/mL (95% CI: 3996.8, 5556.8). Comparable responses were observed in both the initially seronegative and seropositive subjects.
In three combined clinical studies 1022 initially seronegative subjects received 2 doses of VAQTA alone or concomitantly with other vaccines. Of the seronegative subjects, 99.9% achieved an anti-HAV titer ≥10 mIU/mL (95% CI: 99.5%, 100%) and an anti-HAV GMT of 5392.1 mIU/mL (95% CI: 4996.5, 5819.0) 4 weeks following dose 2 of VAQTA.
Children/Adolescents — 2 Years through 18 Years of Age
Immunogenicity data were combined from eleven randomized clinical studies in children and adolescents 2 through 18 years of age who received VAQTA (25U/0.5 mL). These included administration of VAQTA in varying doses and regimens (N=404 received 25U/0.5 mL), the Monroe Efficacy Study (N=973), and comparison studies for process and formulation changes (N=1238). The race distribution of the study subjects who received at least one dose of VAQTA in these studies was as follows: 84.8% Caucasian; 10.6% American Indian; 2.3% African-American; 1.5% Hispanic-American; 0.6% other; 0.2% Oriental. The distribution of subjects by gender was 51.2% male and 48.8% female. The proportions of subjects who seroconverted 4 weeks after the first and second doses administered 6 months apart were 97% (n=1230; 95% CI: 96%, 98%) and 100% (n=1057; 95% CI: 99.5%, 100%) of subjects with anti-HAV GMTs of 43 mIU/mL (95% CI: 40, 45) and 10,077 mIU/mL (95% CI: 9394, 10,810), respectively.
Adults — 19 Years of Age and Older
Immunogenicity data were combined from five randomized clinical studies in adults 19 years of age and older who received VAQTA (50U/1-mL). One single-blind study evaluated doses of VAQTA with varying amounts of viral antigen and/or alum content in healthy adults ≥170 pounds and ≥30 years of age (N=208 adults administered 50U/1-mL dose). One open-label study evaluated VAQTA given with immune globulin or alone (N=164 adults who received VAQTA alone). A third study was single-blind and evaluated 3 different lots of VAQTA (N=1112). The fourth study was single-blind and evaluated doses of VAQTA with varying amounts of viral antigen in healthy adults ≥170 pounds and ≥30 years of age (N=159 adults administered the 50U/1-mL dose). The fifth study was an open-label study to evaluate various regimens for time of administration of the booster dose of VAQTA (6, 12, and 18 months post dose 1, N=354). The race distribution of the study subjects who received at least one dose of VAQTA in these studies was as follows: 93.2% Caucasian; 2.5% African-American; 2.1% Hispanic-American; 1.4% Oriental; 0.5% other; 0.3% American Indian. The distribution of subjects by gender was 44.8% male and 55.2% female. The proportion of subjects who seroconverted 4 weeks after the first and second doses administered 6 months apart was 95% (n=1411; 95% CI: 94%, 96%) and 99.9% (n=1244; 95% CI: 99.4%, 100%) with GMTs of 37 mIU/mL (95% CI: 35, 38) and 6013 mIU/mL (95% CI: 5592, 6467), respectively. Furthermore, at 2 weeks postvaccination, 69.2% (n=744; 95% CI: 65.7%, 72.5%) of adults seroconverted with an anti-HAV GMT of 16 mIU/mL after a single dose of VAQTA.
14.3 Timing of Booster Dose Administration
Children/Adolescents — 2 through 18 Years of Age
In the Monroe Efficacy Study, children were administered a second dose of VAQTA (25U/0.5 mL) 6, 12, or 18 months following the initial dose. For subjects who received both doses of VAQTA, the GMTs and proportions of subjects who seroconverted 4 weeks after the booster dose administered 6, 12, and 18 months after the first dose are presented in Table 9.
Table 9: Children/Adolescents from the Monroe Efficacy Study Seroconversion Rates (%) and Geometric Mean Titers (GMT) for Cohorts of Initially Seronegative Vaccinees at the Time of the Booster(25U) and 4 Weeks Later Months Following Initial 25U Dose Cohort* (n=960)
0 and 6 MonthsCohort* (n=35)
0 and 12 MonthsCohort* (n=39)
0 and 18 MonthsSeroconversion Rate
GMT (mIU/mL) (95% CI)- *
- Blood samples were taken at prebooster and postbooster time points.
6 97%
107 (98, 117)— — 7 100%
10433 (9681, 11243)— — 12 — 91%
48 (33, 71)— 13 — 100%
12308 (9337, 16226)— 18 — — 90%
50 (28, 89)19 — — 100%
9591 (7613, 12082)Adults — 19 years of age and older
Among the 5 randomized clinical studies in adults 19 years of age and older described in Section 14.2, there were additional data in which a booster dose of VAQTA (50U/1-mL) was administered 12 or 18 months after the first dose. For subjects in these studies who received both doses of VAQTA, the proportions who seroconverted 4 weeks after the booster dose administered 6, 12, and 18 months after the first dose were 100% of 1201 subjects, 98% of 91 subjects, and 100% of 84 subjects, respectively. GMTs in mIU/mL one month after the subjects received the booster dose at 6, 12, or 18 months after the primary dose were 5987 mIU/mL (95% CI: 5561, 6445), 4896 mIU/mL (95% CI: 3589, 6679), and 6043 mIU/mL (95% CI: 4687, 7793), respectively.
14.4 Duration of Immune Response
In follow-up of subjects in The Monroe Efficacy Study, in children (≥2 years of age) and adolescents who received two doses (25U) of VAQTA, detectable levels of anti-HAV antibodies (≥10 mIU/mL) were present in 100% of subjects for at least 10 years postvaccination. In subjects who received VAQTA at 0 and 6 months, the GMT was 819 mIU/mL (n=175) at 2.5 to 3.5 years and 505 mIU/mL (n=174) at 5 to 6 years, and 574 mIU/mL (n=114) at 10 years postvaccination. In subjects who received VAQTA at 0 and 12 months, the GMT was 2224 mIU/mL (n=49) at 2.5 to 3.5 years, 1191 mIU/mL (n=47) at 5 to 6 years, and 1005 mIU/mL (n=36) at 10 years postvaccination. In subjects who received VAQTA at 0 and 18 months, the GMT was 2501 mIU/mL (n=53) at 2.5 to 3.5 years, 1614 mIU/mL (n=56) at 5 to 6 years, and 1507 mIU/mL (n=41) at 10 years postvaccination.
In adults that were administered VAQTA at 0 and 6 months, the hepatitis A antibody response to date has been shown to persist at least 6 years. Detectable levels of anti-HAV antibodies (≥10 mIU/mL) were present in 100% (378/378) of subjects with a GMT of 1734 mIU/mL at 1 year, 99.2% (252/254) of subjects with a GMT of 687 mIU/mL at 2 to 3 years, 99.1% (219/221) of subjects with a GMT of 605 mIU/mL at 4 years, and 99.4% (170/171) of subjects with a GMT of 684 mIU/mL at 6 years postvaccination.
The total duration of the protective effect of VAQTA in healthy vaccinees is unknown at present.
14.5 Concomitant Administration of VAQTA and Immune Globulin
The concurrent use of VAQTA (50U) and immune globulin (IG, 0.06 mL/kg) was evaluated in an open-label, randomized clinical study involving 294 healthy adults 18 to 39 years of age. Adults were randomized to receive 2 doses of VAQTA 24 weeks apart (N=129), the first dose of VAQTA concomitant with a dose of IG followed by the second dose of VAQTA alone 24 weeks later (N=135), or IG alone (N=30). The race distribution of the study subjects who received at least one dose of VAQTA or IG in this study was as follows: 92.3% Caucasian; 4.0% Hispanic-American; 3.0% African-American; 0.3% Native American; 0.3% Asian/Pacific. The distribution of subjects by gender was 28.7% male and 71.3% female. Table 10 provides seroconversion rates and GMTs at 4 and 24 weeks after the first dose in each treatment group and at one month after a booster dose of VAQTA (administered at 24 weeks) [see Drug Interactions (7.2)].
Table 10: Seroconversion Rates (%) and Geometric Mean Titers (GMT) After Vaccination with VAQTA Plus IG, VAQTA Alone, and IG Alone VAQTA plus IG VAQTA IG Weeks Seroconversion Rate
GMT (mIU/mL) (95% CI)N/A = Not Applicable. 4 100%
42 (39, 45)
(n=129)96%
38 (33, 42)
(n=135)87%
19 (15, 23)
(n=30)24 92%
83 (65, 105)
(n=125)97%*
137* (112, 169)
(n=132)0%
Undetectable†
(n=28)28 100%
4872 (3716, 6388)
(n=114)100%
6498 (5111, 8261)
(n=128)N/A 14.6 Interchangeability of the Booster Dose
A randomized, double-blind clinical study in 537 healthy adults, 18 to 83 years of age, evaluated the immune response to a booster dose of VAQTA and HAVRIX given at 6 or 12 months following an initial dose of HAVRIX. Subjects were randomized to receive VAQTA (50U) as a booster dose 6 months (N=232) or 12 months (N=124) following an initial dose of HAVRIX or HAVRIX (1440 EL. U) as a booster dose 6 months (N=118) or 12 months (N=63) following an initial dose of HAVRIX. The race distribution of the study subjects who received the booster dose of VAQTA or HAVRIX in this study was as follows: 87.2% Caucasian; 8.0% African-American; 1.9% Hispanic-American; 1.3% Oriental; 0.9% Asian; 0.4% Indian; 0.4% other. The distribution of subjects by gender was 44.9% male and 55.1% female. When VAQTA was given as a booster dose following HAVRIX, the vaccine produced an adequate immune response (see Table 11) [see Dosage and Administration (2.1)].
Table 11: Seropositivity Rate, Booster Response Rate* and Geometric Mean Titer 4 Weeks Following a Booster Dose of VAQTA or HAVRIX Administered 6 to 12 Months After First Dose of HAVRIX† First Dose Booster Dose Seropositivity Rate Booster Response Rate* Geometric Mean Titer HAVRIX
1440 EL.U.VAQTA
50 U99.7% (n=313) 86.1% (n=310) 3272 (n=313) HAVRIX
1440 EL.U.HAVRIX
1440 EL.U.99.3% (n=151) 80.1% (n=151) 2423 (n=151) 14.7 Immune Response to Concomitantly Administered Vaccines
Clinical Studies of VAQTA with M-M-R II, VARIVAX, and Tripedia
In the clinical trial in which children 12 months of age received the first dose of VAQTA concomitantly with M-M-R II and VARIVAX described in Section 14.2, rates of seroprotection to hepatitis A were similar between the two groups who received VAQTA with or without M-M-R II and VARIVAX. Measles, mumps, and rubella immune responses were tested in 241 subjects, 263 subjects, and 270 subjects, respectively. Seropositivity rates were 98.8% [95% CI: 96.4%, 99.7%] for measles, 99.6% [95% CI: 97.9%, 100%] for mumps, and 100% [95% CI: 98.6%, 100%] for rubella, which were similar to observed historical rates (seropositivity rates 99% for all three antigens, with lower bound of the 95% CI >89%) following vaccination with a first dose of M-M-R II in this age group. Data from this study were insufficient to adequately assess the immune response to VARIVAX administered concomitantly with VAQTA. In this same study, the second dose of VAQTA at 18 months of age was given with or without Tripedia (DTaP). Seropositivity rates for diphtheria and tetanus were similar to those in historical controls. However, data from this study were insufficient to assess the pertussis response of DTaP when administered with VAQTA. Rates of seroprotection to hepatitis A were similar between the two groups who received VAQTA with or without M-M-R II and VARIVAX, and between the two groups who received VAQTA with or without DTaP.
Clinical Studies of VAQTA with ProQuad and Prevnar
In the clinical trial of concomitant use of VAQTA with ProQuad and pneumococcal 7-valent conjugate vaccine in children 12 to 15 months of age described in Section 14.2, the antibody GMTs for S. pneumoniae types 4, 6B, 9V, 14, 18C, 19F, and 23F 6 weeks after vaccination with pneumococcal 7-valent conjugate vaccine administered concomitantly with ProQuad and VAQTA were non-inferior as compared to GMTs observed in the group given pneumococcal 7-valent conjugate vaccine with ProQuad alone (the lower bounds of the 95% CI around the fold-difference for the 7 serotypes excluded 0.5). For the varicella component of ProQuad, in subjects with baseline antibody titers <1.25 gpELISA units/mL, the proportion with a titer ≥5 gpELISA units/mL 6 weeks after their first dose of ProQuad was non-inferior (defined as -10 percentage point change) when ProQuad was administered with VAQTA and pneumococcal 7-valent conjugate vaccine as compared to the proportion with a titer ≥5 gpELISA units/mL when ProQuad was administered with pneumococcal 7-valent conjugate vaccine alone (difference in seroprotection rate -5.1% [95% CI: -9.3, -1.4%]). Hepatitis A responses were similar when compared between the two groups who received VAQTA with or without ProQuad and pneumococcal 7-valent conjugate vaccine. Seroconversion rates and antibody titers for varicella and S. pneumoniae types 4, 6B, 9V, 14, 18C, 19F, and 23F were similar between groups at 6 weeks postvaccination.
Clinical Studies of VAQTA with INFANRIX and PedvaxHIB
In the clinical trial of concomitant administration of VAQTA with INFANRIX and PedvaxHIB in children 15 months of age, described in Section 14.2, when the first dose of VAQTA was administered concomitantly with either INFANRIX and PedvaxHIB or PedvaxHIB, there was no interference in immune response to hepatitis A as measured by seropositivity rates after dose 2 of VAQTA compared to administration of both doses of VAQTA alone. When dose 1 of VAQTA was administered concomitantly with either PedvaxHIB and INFANRIX or PedvaxHIB, there was no interference in immune response to Haemophilus influenzae b (as measured by the proportion of subjects who attained an anti-polyribosylribitol phosphate antibody titer >1.0 mcg/mL at 4 weeks after vaccination), compared to subjects receiving either PedvaxHIB and INFANRIX or PedvaxHIB. When VAQTA was administered concomitantly with INFANRIX and PedvaxHIB, there was no interference in immune responses at 4 weeks after vaccination to the pertussis antigens (PT, FHA, or pertactin, as measured by GMTs) and no interference in immune responses to diphtheria toxoid or tetanus toxoid (as measured by the proportion of subjects achieving an antibody titer >0.1 IU/mL) compared to administration of INFANRIX and PedvaxHIB.
Clinical Studies of VAQTA with Typhoid Vi Polysaccharide Vaccine and Yellow Fever Vaccine, Live Attenuated
In the clinical trial of concomitant use of VAQTA with typhoid Vi polysaccharide and yellow fever vaccines in adults 18-54 years of age described in Section 6.1, the antibody response rates for typhoid Vi polysaccharide and yellow fever were adequate when typhoid Vi polysaccharide and yellow fever vaccines were administered concomitantly with (N=80) and nonconcomitantly without VAQTA (N=80). The seropositivity rate for hepatitis A when VAQTA, typhoid Vi polysaccharide, and yellow fever vaccines were administered concomitantly was generally similar to when VAQTA was given alone [see Drug Interactions (7.1)].
Data are insufficient to assess the immune response to VAQTA and poliovirus vaccine when administered concomitantly.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
VAQTA is available in single-dose vials and prefilled Luer-Lok® syringes.
Pediatric/Adolescent Formulations
25U/0.5 mL in single-dose vials and prefilled Luer-Lok® syringes.
NDC 0006-4831-41 – box of ten 0.5-mL single dose vials.
NDC 0006-4095-02 – carton of ten 0.5-mL prefilled single-dose Luer-Lok® syringes with tip caps. -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Information for Vaccine Recipients and Parents or Guardians
- Inform the patient, parent or guardian of the potential benefits and risks of the vaccine.
- Question the vaccine recipient, parent, or guardian about the occurrence of any symptoms and/or signs of an adverse reaction after a previous dose of hepatitis A vaccine.
- Inform the patient, parent, or guardian about the potential for adverse events that have been temporally associated with administration of VAQTA.
- Tell the patient, parent, or guardian accompanying the recipient, to report adverse events to the physician or clinic where the vaccine was administered.
- Prior to vaccination, give the patient, parent, or guardian the Vaccine Information Statements which are required by the National Childhood Vaccine Injury Act of 1986. These materials are available free of charge at the Centers for Disease Control and Prevention (CDC) website (www.cdc.gov/vaccines).
- Tell the patient, parent, or guardian that the United States Department of Health and Human Services has established a Vaccine Adverse Event Reporting System (VAERS) to accept all reports of suspected adverse events after the administration of any vaccine, including but not limited to the reporting of events required by the National Childhood Vaccine Injury Act of 1986. The VAERS toll-free number is 1-800-822-7967. Reporting forms may also be obtained at the VAERS website at (www.//vaers.hhs.gov/).
-
SPL UNCLASSIFIED SECTION
Manuf. and Dist. by: Merck Sharp & Dohme LLC
Rahway, NJ 07065, USAFor patent information: www.msd.com/research/patent
The trademarks depicted herein are owned by their respective companies.
Copyright ©1996-2023 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates.
All rights reserved.uspi-v251-i-2304r020
-
PATIENT PACKAGE INSERT
Patient Information
VAQTA® (pronounced “vac-ta”)
(Hepatitis A Vaccine, Purified Inactivated)
This is a summary of information about VAQTA®. Read this information carefully before you or your child receives each dose of VAQTA. If you have any questions about VAQTA after reading this leaflet, you should ask your doctor. This information does not take the place of talking about VAQTA with your doctor or healthcare provider.
What is VAQTA?- VAQTA is a vaccine that helps prevent Hepatitis A. Hepatitis A is an infection of the liver.
- It is a vaccine for people 12 months of age and older.
What do you need to know about VAQTA? - You cannot get Hepatitis A from VAQTA.
- VAQTA will not prevent other types of Hepatitis, such as Hepatitis B.
- VAQTA may not be effective in everyone who gets the shot.
- VAQTA is not a treatment for those who already have Hepatitis A.
Who should not get VAQTA? Do not get VAQTA if you or your child: - have had an allergic reaction to any ingredients of VAQTA, including neomycin. (See section titled “What is in VAQTA?” at the end of this leaflet.)
- have had an allergic reaction to a previous dose of any Hepatitis A vaccine.
What should you tell the doctor or healthcare provider before getting VAQTA? Tell your doctor or healthcare provider if you or your child: - are allergic to latex. The vial stopper and the syringe plunger stopper and tip cap contain dry natural latex rubber. Latex may cause severe allergic reactions in some people.
- are pregnant or planning to get pregnant.
- are breast-feeding.
- have immune problems, like HIV or cancer.
If you have already gotten a Hepatitis A shot, talk to your doctor or healthcare provider to see if VAQTA is right for you.
The doctor will help decide if you or your child should get the shot.
What are the possible side effects of VAQTA? The most common side effects seen with VAQTA are: - pain, soreness, redness, swelling, and warmth where you or your child got the shot
- headache (adults 19 years and older)
- fever (children 12 to 23 months)
If you or your child have any of the following problems, tell your doctor right away because these may be signs of an allergic reaction: - trouble breathing
- wheezing
- hives
- rash
If you or your child has any side effects that worry you or seem to get worse, tell your doctor or healthcare provider right away.
There may be other side effects that are not listed. For more information, ask your doctor or healthcare provider.
You may report any side effects to your or your child’s doctor or directly to the Vaccine Adverse Event Reporting System (VAERS) at 1-800-822-7967 or www.vaers.hhs.gov, or to Merck Sharp & Dohme LLC at 1-877-888-4231.
How is VAQTA given?
- It is a shot given in the arm or thigh.
- VAQTA is given in two doses on two different dates.
- the 1st shot is given any time after 12 months of age.
- the 2nd shot is given 6-18 months after the first shot.
- If you miss the 2nd shot or if you get your 2nd shot too soon, talk to your doctor or healthcare provider. They will decide what to do.
- How much VAQTA is given?
- Children/Adolescents - 12 months through 18 years of age (0.5 mL dose)
- Adults -19 years of age and older (1.0 mL dose)
Active ingredient: Hepatitis A virus, Inactivated.
Other ingredients: Aluminum hydroxyphosphate sulphate, sodium borate, sodium chloride, water.
This vaccine contains a trace amount of neomycin.
The vial stopper, syringe plunger stopper and tip cap contain natural latex rubber.
VAQTA does not have any preservatives in it.
For more information, ask your doctor or healthcare provider. Keep this information in case you have questions later. -
SPL UNCLASSIFIED SECTION
Manuf. and Dist. by: Merck Sharp & Dohme LLC
Rahway, NJ 07065, USAFor patent information: www.msd.com/research/patent
Copyright © 2006-2023 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates.
All rights reserved.usppi-v251-i-2304r001
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 04/2023
-
PRINCIPAL DISPLAY PANEL - 0.5 mL Vials Carton
NDC 0006-4831-41
10 Single-dose ~25U/0.5-mL Vials(HEPATITIS A VACCINE, INACTIVATED)
VAQTA®
PEDIATRIC/ADOLESCENT0.5 mL vial contains approximately 25U of hepatitis A virus antigen on an Amorphous aluminum
hydroxyphosphate sulfate adjuvant. Hepatitis A virus is grown in cell culture in human MRC-5
diploid fibroblasts.Rx only

-
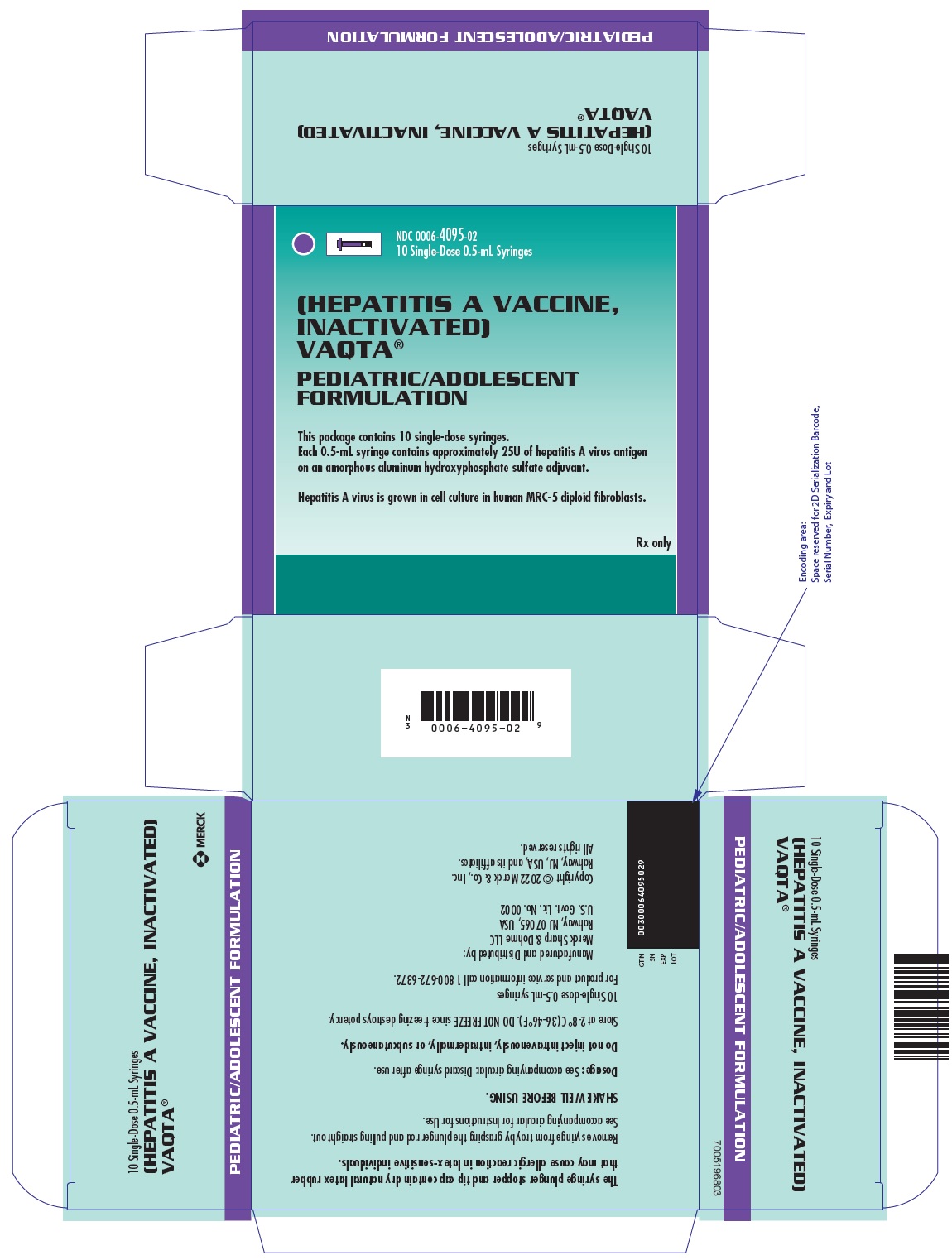
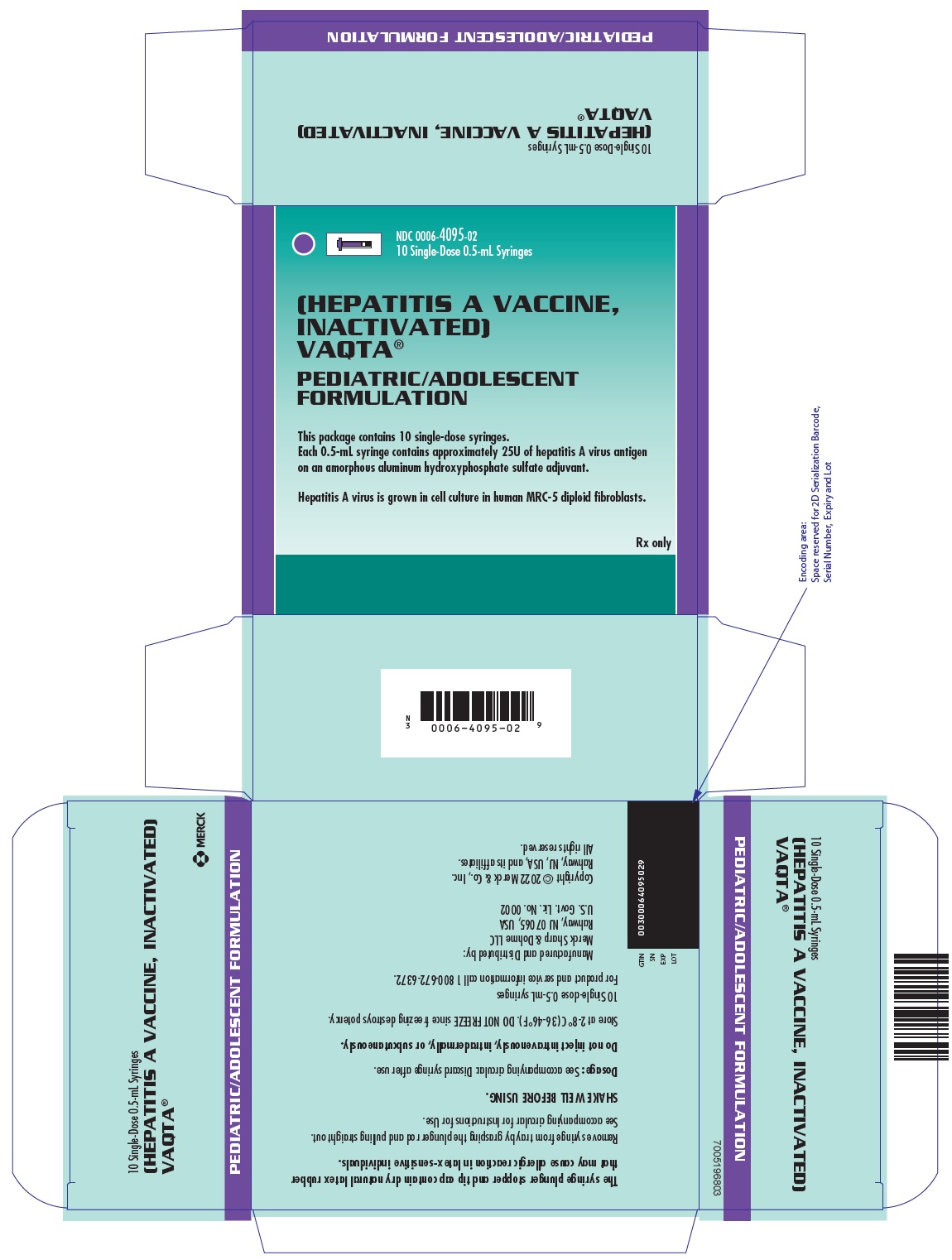
PRINCIPAL DISPLAY PANEL - 0.5 mL Syringes Carton
NDC 0006-4095-02
10 Single-Dose 0.5-mL Syringes[HEPATITIS A VACCINE,
INACTIVATED]
VAQTA®PEDIATRIC/ADOLESCENT
FORMULATIONThis package contains 10 single-dose syringes.
Each 0.5-mL syringe contains approximately 25U of hepatitis A virus antigen
on an amorphous aluminum hydroxyphosphate sulfate adjuvant.Hepatitis A virus is grown in cell culture in human MRC-5 diploid fibroblasts.
Rx only
-
PRINCIPAL DISPLAY PANEL - 1 mL Vials Carton
NDC 0006-4841-41
10 Single-dose
~50U/1-mL Vials(HEPATITIS A VACCINE, INACTIVATED)
VAQTA®
ADULT FORMULATION1 mL vial contains approximately 50U of hepatitis A virus antigen on an Amorphous aluminum
hydroxyphosphate sulfate adjuvant. Hepatitis A virus is grown in cell culture in human MRC-5
diploid fibroblasts.Rx only

-
PRINCIPAL DISPLAY PANEL - 1 mL Syringes Carton
NDC 0006-4096-02
10 Single-Dose 1-mL Syringes[HEPATITIS A VACCINE,
INACTIVATED]
VAQTA®ADULT FORMULATION
This package contains 10 single-dose syringes.
Each 1-mL syringe contains approximately 50U of hepatitis A virus antigen
on an amorphous aluminum hydroxyphosphate sulfate adjuvant.Hepatitis A virus is grown in cell culture in human MRC-5 diploid fibroblasts.
Rx only

-
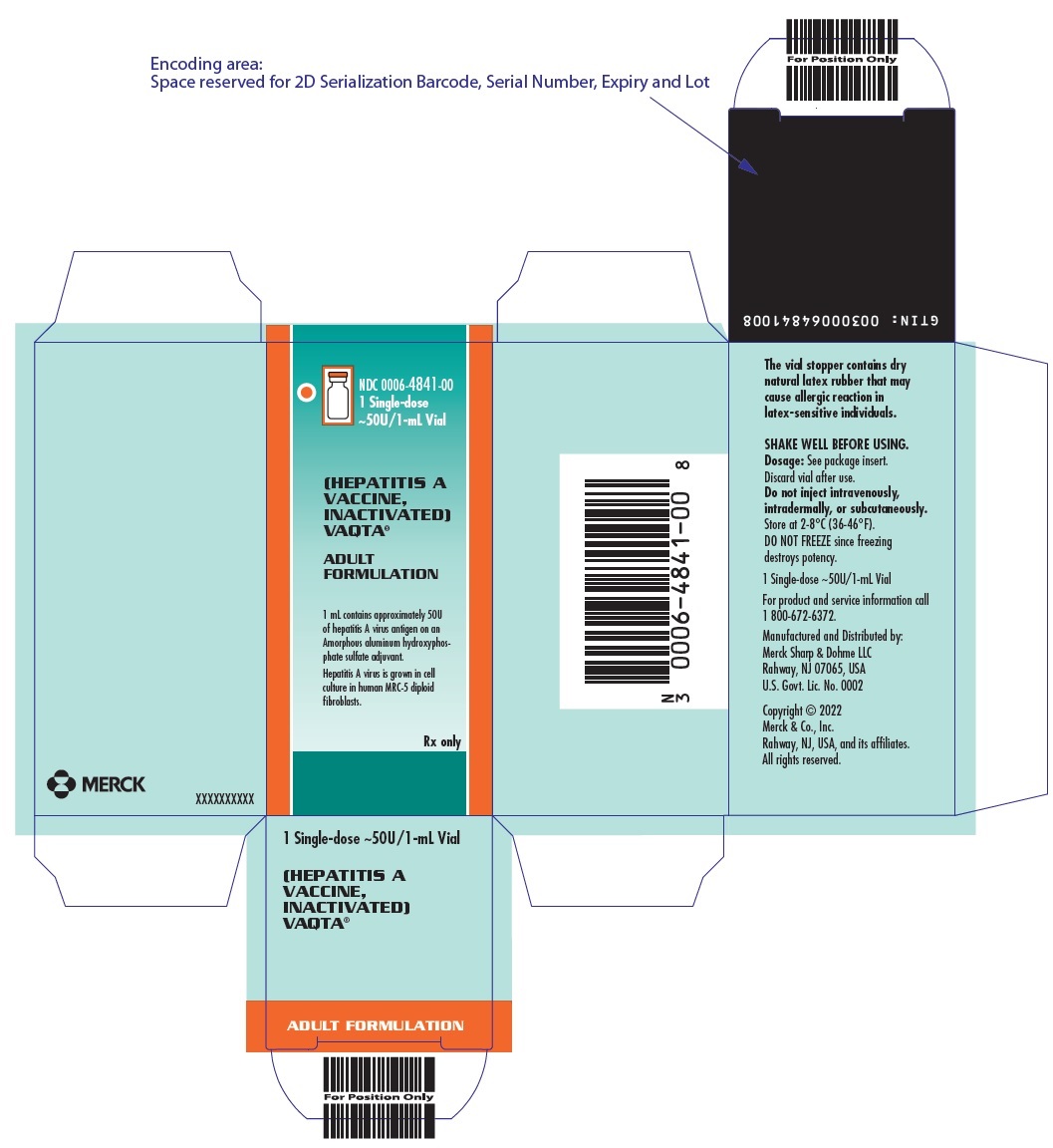
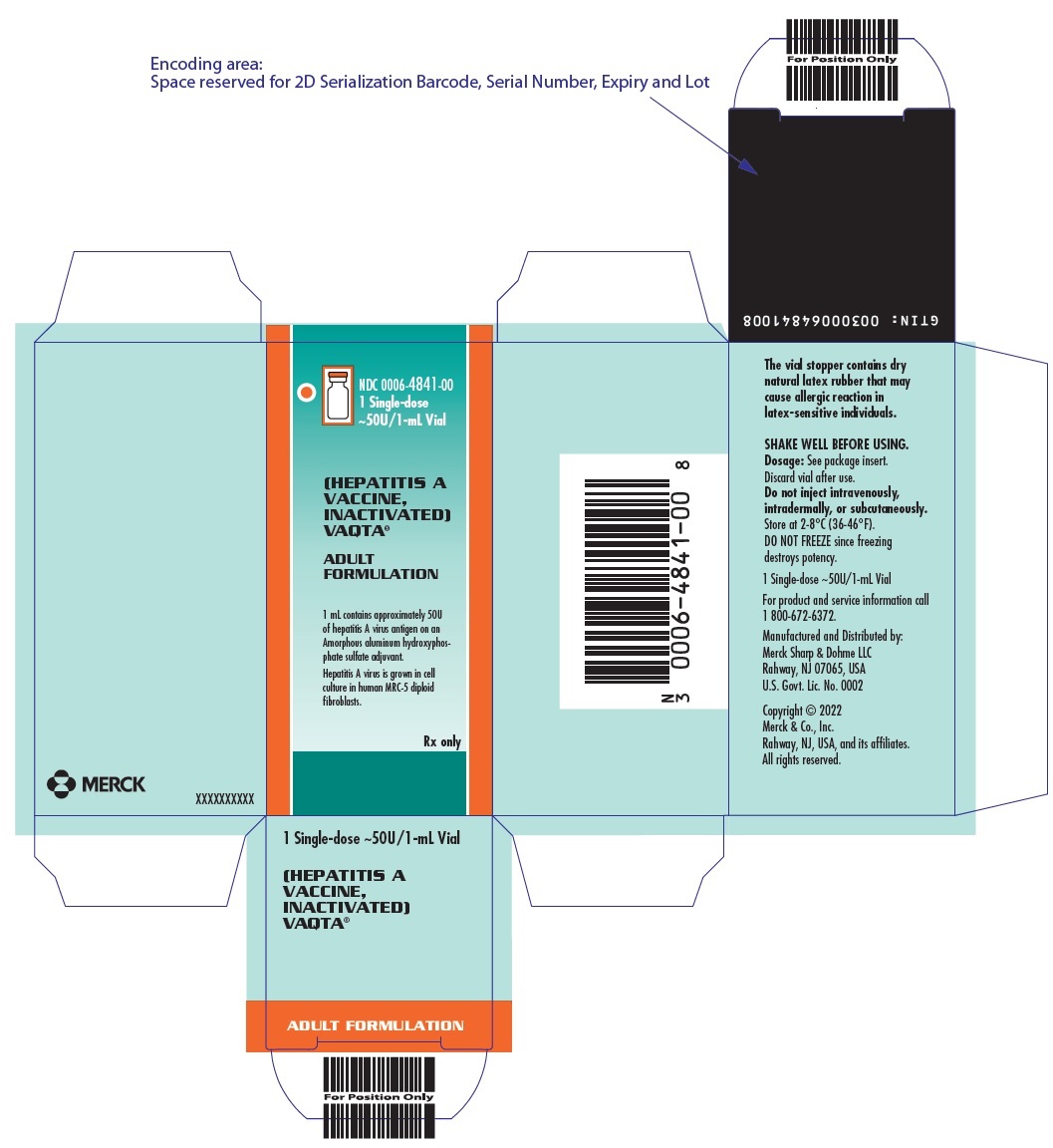
PRINCIPAL DISPLAY PANEL - 1 mL Vial Carton
NDC 0006-4841-00
1 Single-dose
~50U/1-mL Vial(HEPATITIS A
VACCINE,
INACTIVATED)
VAQTA®ADULT
FORMULATION1 mL contains approximately 50U
of hepatitis A virus antigen on an
Amorphous aluminum hydroxyphos-
phate sulfate adjuvant.Hepatitis A virus is grown in cell
culture in human MRC-5 diploid
fibroblasts.Rx only
-
INGREDIENTS AND APPEARANCE
VAQTA
hepatitis a vaccine, inactivated injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC:0006-4831 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HEPATITIS A VIRUS STRAIN CR 326F ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: Q04Q922K9Q) (HEPATITIS A VIRUS STRAIN CR 326F ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:Q04Q922K9Q) HEPATITIS A VIRUS STRAIN CR 326F ANTIGEN (FORMALDEHYDE INACTIVATED) 25 [iU] in 0.5 mL Inactive Ingredients Ingredient Name Strength SODIUM BORATE (UNII: 91MBZ8H3QO) 35 ug in 0.5 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Other Ingredients Ingredient Kind Ingredient Name Quantity ADJV ALUMINUM HYDROXYPHOSPHATE SULFATE (UNII: F41V936QZM) 0.225 mg in 0.5 mL Product Characteristics Color WHITE (slightly opaque, white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0006-4831-41 10 in 1 CARTON 1 NDC:0006-4831-01 0.5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103606 03/29/1996 VAQTA
hepatitis a vaccine, inactivated injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC:0006-4095 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HEPATITIS A VIRUS STRAIN CR 326F ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: Q04Q922K9Q) (HEPATITIS A VIRUS STRAIN CR 326F ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:Q04Q922K9Q) HEPATITIS A VIRUS STRAIN CR 326F ANTIGEN (FORMALDEHYDE INACTIVATED) 25 [iU] in 0.5 mL Inactive Ingredients Ingredient Name Strength SODIUM BORATE (UNII: 91MBZ8H3QO) 35 ug in 0.5 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Other Ingredients Ingredient Kind Ingredient Name Quantity ADJV ALUMINUM HYDROXYPHOSPHATE SULFATE (UNII: F41V936QZM) 0.225 mg in 0.5 mL Product Characteristics Color WHITE (slightly opaque, white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0006-4095-02 10 in 1 CARTON 1 NDC:0006-4095-01 0.5 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103606 03/29/1996 VAQTA
hepatitis a vaccine, inactivated injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC:0006-4841 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HEPATITIS A VIRUS STRAIN CR 326F ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: Q04Q922K9Q) (HEPATITIS A VIRUS STRAIN CR 326F ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:Q04Q922K9Q) HEPATITIS A VIRUS STRAIN CR 326F ANTIGEN (FORMALDEHYDE INACTIVATED) 50 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM BORATE (UNII: 91MBZ8H3QO) 70 ug in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Other Ingredients Ingredient Kind Ingredient Name Quantity ADJV ALUMINUM HYDROXYPHOSPHATE SULFATE (UNII: F41V936QZM) 0.45 mg in 1 mL Product Characteristics Color WHITE (slightly opaque, white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0006-4841-41 10 in 1 CARTON 1 NDC:0006-4841-01 1 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product 2 NDC:0006-4841-00 1 in 1 CARTON 2 NDC:0006-4841-01 1 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103606 03/29/1996 VAQTA
hepatitis a vaccine, inactivated injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC:0006-4096 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HEPATITIS A VIRUS STRAIN CR 326F ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: Q04Q922K9Q) (HEPATITIS A VIRUS STRAIN CR 326F ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:Q04Q922K9Q) HEPATITIS A VIRUS STRAIN CR 326F ANTIGEN (FORMALDEHYDE INACTIVATED) 50 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM BORATE (UNII: 91MBZ8H3QO) 70 ug in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Other Ingredients Ingredient Kind Ingredient Name Quantity ADJV ALUMINUM HYDROXYPHOSPHATE SULFATE (UNII: F41V936QZM) 0.45 mg in 1 mL Product Characteristics Color WHITE (slightly opaque, white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0006-4096-02 10 in 1 CARTON 1 NDC:0006-4096-01 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103606 03/29/1996 Labeler - Merck Sharp & Dohme LLC (118446553)