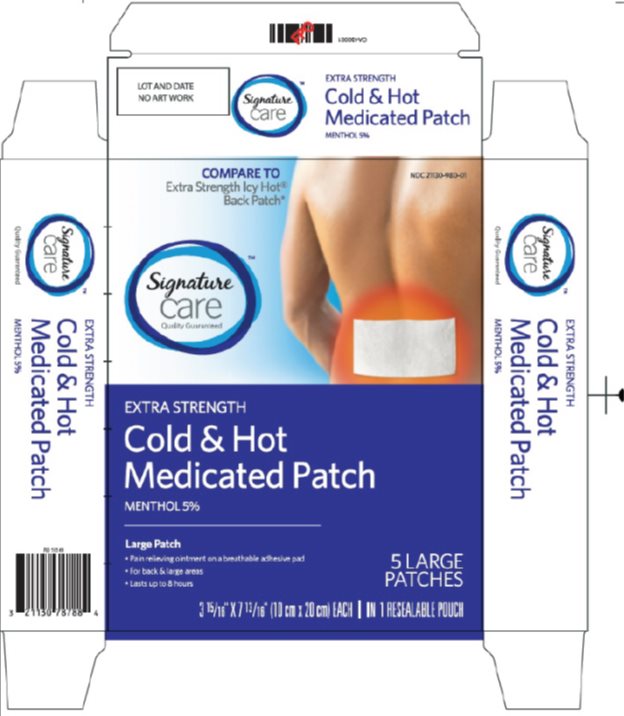
Label: SIGNATURE CARE COLD AND HOT MEDICATED- menthol patch
- NDC Code(s): 21130-980-01
- Packager: Safeway
- This is a repackaged label.
- Source NDC Code(s): 64032-8125
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
When using this product
- use only as directed
- avoid contact with eyes or on mucous membranes
- do not apply to wounds or to damaged or very sensitive skin
- do not bandage tightly or use with a heating pad
-
Directions
- adults and children 12 years and over: apply patch to affected area as needed but not more than 4 times daily
- children under 12 years: ask a doctor
- for easy application; partially peel back protective film and apply exposed patch to site of pain. Carefully remove remaining film while pressing patch to skin.
- Inactive ingredients
- Package/Label Principal Display Panel
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SIGNATURE CARE COLD AND HOT MEDICATED
menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:21130-980(NDC:64032-8125) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 50 mg Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) CASTOR OIL (UNII: D5340Y2I9G) DIHYDROXYALUMINUM AMINOACETATE (UNII: DO250MG0W6) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) KAOLIN (UNII: 24H4NWX5CO) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POLYACRYLIC ACID (250000 MW) (UNII: 9G2MAD7J6W) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) TARTARIC ACID (UNII: W4888I119H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21130-980-01 1 in 1 CARTON 09/01/2015 1 5 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 09/01/2015 Labeler - Safeway (009137209)