Label: CLARITHROMYCIN tablet, film coated
- NDC Code(s): 82804-141-28
- Packager: Proficient Rx LP
- This is a repackaged label.
- Source NDC Code(s): 65862-226
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CLARITHROMYCIN TABLETS safely and effectively. See full prescribing information for CLARITHROMYCIN TABLETS. CLARITHROMYCIN ...These highlights do not include all the information needed to use CLARITHROMYCIN TABLETS safely and effectively. See full prescribing information for CLARITHROMYCIN TABLETS.
CLARITHROMYCIN tablets, for oral use
Initial U.S. Approval: 1991
INDICATIONS AND USAGE
Clarithromycin tablets are a macrolide antimicrobial indicated for mild to moderate infections caused by designated, susceptible bacteria in the following:
- •
- Acute Bacterial Exacerbation of Chronic Bronchitis in Adults (1.1)
- •
- Acute Maxillary Sinusitis (1.2)
- •
- Community-Acquired Pneumonia (1.3)
- •
- Pharyngitis/Tonsillitis (1.4)
- •
- Uncomplicated Skin and Skin Structure Infections (1.5)
- •
- Acute Otitis Media in Pediatric Patients (1.6)
- •
- Treatment and Prophylaxis of Disseminated Mycobacterial Infections (1.7)
- •
- Helicobacter pylori Infection and Duodenal Ulcer Disease in Adults (1.8)
Limitations of Use
To reduce the development of drug-resistant bacteria and maintain the effectiveness of clarithromycin tablets and other antibacterial drugs, clarithromycin tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. (1.9)DOSAGE AND ADMINISTRATION
- •
- Adults: Clarithromycin tablets 250 mg or 500 mg every 12 hours for 7 to 14 days (2.2)
- •
- H. pylori eradication (in combination with lansoprazole/amoxicillin, omeprazole/amoxicillin, or omeprazole): Clarithromycin tablets 500 mg every 8 or 12 hours for 10 to 14 days. See full prescribing information (FPI) for additional information. (2.3)
- •
- Pediatric Patients: Clarithromycin tablets 15 mg/kg/day divided every 12 hours for 10 days (2.4)
- •
- Mycobacterial Infections: Clarithromycin tablets 500 mg every 12 hours; clarithromycin tablets 7.5 mg/kg up to 500 mg every 12 hours in pediatric patients (2.5)
- •
- Reduce dose in moderate renal impairment with concomitant atazanavir or ritonavir-containing regimens and in severe renal impairment (2.6)
DOSAGE FORMS AND STRENGTHS
- •
- Tablets: 250 mg and 500 mg (3)
CONTRAINDICATIONS
- •
- Hypersensitivity to clarithromycin or any macrolide drug (4.1)
- •
- Cisapride and pimozide (4.2)
- •
- History of cholestatic jaundice/hepatic dysfunction with use of clarithromycin (4.3)
- •
- Colchicine in renal or hepatic impairment (4.4)
- •
- Lomitapide, lovastatin, and simvastatin (4.5)
- •
- Ergot alkaloids (ergotamine or dihydroergotamine) (4.6)
- •
- Lurasidone (4.7)
WARNINGS AND PRECAUTIONS
- •
- Severe Acute Hypersensitivity Reactions: Discontinue clarithromycin if occurs (5.1)
- •
- QT Prolongation: Avoid clarithromycin in patients with known QT prolongation or receiving drugs known to prolong the QT interval, ventricular arrhythmia (torsades de pointes), hypokalemia /hypomagnesemia, significant bradycardia, or taking Class IA or III antiarrhythmics (5.2)
- •
- Hepatotoxicity: Discontinue if signs and symptoms of hepatitis occur (5.3)
- •
- Serious adverse reactions can occur due to drug interactions of clarithromycin with colchicine, some lipid-lowering agents, some calcium channel blockers, and other drugs (5.4)
- •
- Risk of all-cause mortality one year or more after the end of treatment in patients with coronary artery disease. Balance this potential risk with the treatment benefits when prescribing clarithromycin in these patients (5.5)
- •
- Clostridium difficile associated diarrhea (CDAD): Evaluate if diarrhea occurs (5.6)
- •
- Embryo-Fetal Toxicity: Based on animal findings, clarithromycin is not recommended for use in pregnant women except in clinical circumstances where no alternative therapy is appropriate (5.7)
- •
- Exacerbation of myasthenia gravis has been reported in patients receiving clarithromycin therapy (5.8)
ADVERSE REACTIONS
Most frequent adverse reactions for both adult and pediatric populations in clinical trials: abdominal pain, diarrhea, nausea, vomiting, dysgeusia (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Aurobindo Pharma USA, Inc. at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
Geriatric: Increased risk of torsades de pointes (8.5)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2024
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Acute Bacterial Exacerbation of Chronic Bronchitis
1.2 Acute Maxillary Sinusitis
1.3 Community-Acquired Pneumonia
1.4 Pharyngitis/Tonsillitis
1.5 Uncomplicated Skin and Skin Structure Infections
1.6 Acute Otitis Media
1.7 Treatment and Prophylaxis of Disseminated Mycobacterial Infections
1.8 Helicobacter pylori Infection and Duodenal Ulcer Disease
1.9 Limitations of Use
1.10 Usage
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Adult Dosage
2.3 Combination Dosing Regimens for H. pylori Infection
2.4 Pediatric Dosage
2.5 Dosage Regimens for Mycobacterial Infections
2.6 Dosage Adjustment in Patients with Renal Impairment
2.7 Dosage Adjustment Due to Drug Interactions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity
4.2 Cisapride and Pimozide
4.3 Cholestatic Jaundice/Hepatic Dysfunction
4.4 Colchicine
4.5 Lomitapide, Lovastatin, and Simvastatin
4.6 Ergot Alkaloids
4.7 Lurasidone
4.8 Contraindications for Co-administered Drugs
5 WARNINGS AND PRECAUTIONS
5.1 Severe Acute Hypersensitivity Reactions
5.2 QT Prolongation
5.3 Hepatotoxicity
5.4 Serious Adverse Reactions Due to Concomitant Use with Other Drugs
5.5 All-Cause Mortality in Patients With Coronary Artery Disease 1 to 10 Years After Clarithromycin Exposure
5.6 Clostridium difficile Associated Diarrhea
5.7 Embryo-Fetal Toxicity
5.8 Exacerbation of Myasthenia Gravis
5.9 Development of Drug Resistant Bacteria
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal and Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Mycobacterial Infections
14.2 Otitis Media
14.3 H. pylori Eradication to Decrease the Risk of Duodenal Ulcer Recurrence
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE1.1 Acute Bacterial Exacerbation of Chronic Bronchitis - Clarithromycin tablets are indicated in adults for the treatment of mild to moderate infections caused by susceptible isolates due to ...
1.1 Acute Bacterial Exacerbation of Chronic Bronchitis
Clarithromycin tablets are indicated in adults for the treatment of mild to moderate infections caused by susceptible isolates due to Haemophilus influenzae, Haemophilus parainfluenzae, Moraxella catarrhalis, or Streptococcus pneumoniae [see Indications and Usage (1.9)].
1.2 Acute Maxillary Sinusitis
Clarithromycin tablets are indicated for the treatment of mild to moderate infections caused by susceptible isolates due to Haemophilus influenzae, Moraxella catarrhalis, or Streptococcus pneumoniae [see Indications and Usage (1.9)].
1.3 Community-Acquired Pneumonia
Clarithromycin tablets are indicated [see Indications and Usage (1.9)] for the treatment of mild to moderate infections caused by susceptible isolates due to:
1.4 Pharyngitis/Tonsillitis
Clarithromycin tablets are indicated for the treatment of mild to moderate infections caused by susceptible isolates due to Streptococcus pyogenes as an alternative in individuals who cannot use first line therapy.
1.5 Uncomplicated Skin and Skin Structure Infections
Clarithromycin tablets are indicated for the treatment of mild to moderate infections caused by susceptible isolates due to Staphylococcus aureus, or Streptococcus pyogenes.
1.6 Acute Otitis Media
Clarithromycin tablets are indicated in pediatric patients for the treatment of mild to moderate infections caused by susceptible isolates due to Haemophilus influenzae, Moraxella catarrhalis, or Streptococcus pneumoniae [see Clinical Studies (14.2)].
1.7 Treatment and Prophylaxis of Disseminated Mycobacterial Infections
Clarithromycin tablets are indicated for the treatment of mild to moderate infections caused by susceptible isolates due to Mycobacterium avium or Mycobacterium intracellulare in patients with advanced HIV infection [see Clinical Studies (14.1)].
1.8 Helicobacter pylori Infection and Duodenal Ulcer Disease
Clarithromycin tablets are given in combination with other drugs in adults as described below to eradicate H. pylori. The eradication of H. pylori has been demonstrated to reduce the risk of duodenal ulcer recurrence [see Clinical Studies (14.3)].
- •
- Clarithromycin tablets in combination with amoxicillin and PREVACID (lansoprazole) or PRILOSEC (omeprazole) Delayed-Release Capsules, as triple therapy, are indicated for the treatment of patients with H. pylori infection and duodenal ulcer disease (active or five-year history of duodenal ulcer) to eradicate H. pylori.
- •
- Clarithromycin tablets in combination with PRILOSEC (omeprazole) capsules are indicated for the treatment of patients with an active duodenal ulcer associated with H. pylori infection. Regimens which contain clarithromycin tablets as the single antibacterial agent are more likely to be associated with the development of clarithromycin resistance among patients who fail therapy. Clarithromycin-containing regimens should not be used in patients with known or suspected clarithromycin resistant isolates because the efficacy of treatment is reduced in this setting.
1.9 Limitations of Use
There is resistance to macrolides in certain bacterial infections caused by Streptococcus pneumoniae and Staphylococcus aureus. Susceptibility testing should be performed when clinically indicated.
Close1.10 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of clarithromycin tablets and other antibacterial drugs, clarithromycin tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
-
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Instructions - Clarithromycin tablets may be given with or without food. 2.2 Adult Dosage - The recommended dosages of clarithromycin tablets for the treatment of ...
2.2 Adult Dosage
The recommended dosages of clarithromycin tablets for the treatment of mild to moderate infections in adults are listed in Table 1.
Table 1. Adult Dosage Guidelines Clarithromycin Tablets
Infection
Dosage
(every 12 hours)
Duration
(days)
Acute bacterial exacerbation of chronic bronchitis
250 to 500 mga
7b to 14
Acute maxillary sinusitis
500 mg
14
Community-acquired pneumonia
250 mg
7d to 14
Pharyngitis/Tonsillitis
250 mg
10
Uncomplicated skin and skin structure infections
250 mg
7 to 14
Treatment and prophylaxis of disseminated Mycobacterium avium disease [see Dosage and Administration (2.5)]
500 mge
-
H.pylori eradication to reduce the risk of duodenal ulcer recurrence with amoxicillin and omeprazole or lansoprazole [see Dosage and Administration (2.3)]
500 mg
10 to 14
H.pylori eradication to reduce the risk of duodenal ulcer recurrence with omeprazole [see Dosage and Administration (2.3)]
500 mg every 8 hours
14
a For M. catarrhalis and S. pneumoniae use 250 mg. For H. influenzae and H. parainfluenzae, use 500 mg.
b For H. parainfluenzae, the duration of therapy is 7 days.
d For H. influenzae, the duration of therapy is 7 days.
e Clarithromycin tablets therapy should continue if clinical response is observed. Clarithromycin tablets can be discontinued when the patient is considered at low risk of disseminated infection.
2.3 Combination Dosing Regimens for H. pylori Infection
- •
- Triple therapy: Clarithromycin tablets/lansoprazole/amoxicillin
The recommended adult dosage is 500 mg clarithromycin tablets, 30 mg lansoprazole, and 1 gram amoxicillin, all given every 12 hours for 10 or 14 days [see Indications and Usage (1.8) and Clinical Studies (14.3)].
- •
- Triple therapy: Clarithromycin tablets/omeprazole/amoxicillin
The recommended adult dosage is 500 mg clarithromycin tablets, 20 mg omeprazole, and 1 gram amoxicillin; all given every 12 hours for 10 days. In patients with an ulcer present at the time of initiation of therapy, an additional 18 days of omeprazole 20 mg once daily is recommended for ulcer healing and symptom relief [see Indications and Usage (1.8) and Clinical Studies (14.3)].
The recommended adult dosage is 500 mg clarithromycin tablets given every 8 hours and 40 mg omeprazole given once every morning for 14 days. An additional 14 days of omeprazole 20 mg once daily is recommended for ulcer healing and symptom relief [see Indications and Usage (1.8) and Clinical Studies (14.3)].
2.4 Pediatric Dosage
The recommended daily dosage is 15 mg/kg/day divided every 12 hours for 10 days (up to the adult dose). Refer to dosage regimens for mycobacterial infections in pediatric patients for additional dosage information [see Dosage and Administration (2.5)].
2.5 Dosage Regimens for Mycobacterial Infections
For the treatment of disseminated infection due to Mycobacterium avium complex (MAC), clarithromycin tablets are recommended as the primary agents. Clarithromycin tablets should be used in combination with other antimycobacterial drugs (e.g., ethambutol) that have shown in vitro activity against MAC or clinical benefit in MAC treatment [see Clinical Studies (14.1)].
Adult Patients
For treatment and prophylaxis of mycobacterial infections in adults, the recommended dose of clarithromycin tablets is 500 mg every 12 hours.
Pediatric Patients
For treatment and prophylaxis of mycobacterial infections in pediatric patients, the recommended dose is 7.5 mg/kg every 12 hours up to 500 mg every 12 hours. [See Use in Specific Populations (8.4) and Clinical Studies (14.1)].
Clarithromycin tablets therapy should continue if clinical response is observed. Clarithromycin tablets can be discontinued when the patient is considered at low risk of disseminated infection.
2.6 Dosage Adjustment in Patients with Renal Impairment
See Table 2 for dosage adjustment in patients with moderate or severe renal impairment with or without concomitant atazanavir or ritonavir-containing regimens [see Drug Interactions (7)].
Table 2. Clarithromycin Tablets Dosage Adjustments in Patients with Renal Impairment
Recommended Clarithromycin Tablets Dosage Reduction
Patients with severe renal impairment (CLcr of <30 mL/min)
Reduce the dosage of clarithromycin tablets by 50%
Patients with moderate renal impairment (CLcr of 30 to 60 mL/min) taking concomitant atazanavir or ritonavir-containing regimens
Reduce the dosage of clarithromycin tablets by 50%
Patients with severe renal impairment (CLcr of <30 mL/min) taking concomitant atazanavir or ritonavir-containing regimens
Reduce the dosage of clarithromycin tablets by 75%
Close2.7 Dosage Adjustment Due to Drug Interactions
Decrease the dose of clarithromycin tablets by 50 % when co-administered with atazanavir [see Drug Interactions (7)]. Dosage adjustments for other drugs when co-administered with clarithromycin tablets may be recommended due to drug interactions [see Drug Interactions (7)].
-
3 DOSAGE FORMS AND STRENGTHSClarithromycin tablets USP are available as: • 250 mg: light yellow colored, oval shaped, biconvex film-coated tablets, with ‘D’ debossed on one side and ‘62’ on the other side. • 500 mg ...
Clarithromycin tablets USP are available as:
- •
- 250 mg: light yellow colored, oval shaped, biconvex film-coated tablets, with ‘D’ debossed on one side and ‘62’ on the other side.
- •
- 500 mg: light yellow colored, oval shaped, biconvex film-coated tablets, with ‘D’ debossed on one side and ‘63’ on the other side.
-
4 CONTRAINDICATIONS4.1 Hypersensitivity - Clarithromycin tablets are contraindicated in patients with a known hypersensitivity to clarithromycin, erythromycin, or any of the macrolide antibacterial drugs [see ...
4.1 Hypersensitivity
Clarithromycin tablets are contraindicated in patients with a known hypersensitivity to clarithromycin, erythromycin, or any of the macrolide antibacterial drugs [see Warnings and Precautions (5.1)].
4.2 Cisapride and Pimozide
Concomitant administration of clarithromycin tablets with cisapride and pimozide is contraindicated [see Drug Interactions (7)].
There have been postmarketing reports of drug interactions when clarithromycin is co-administered with cisapride or pimozide, resulting in cardiac arrhythmias (QT prolongation, ventricular tachycardia, ventricular fibrillation, and torsades de pointes) most likely due to inhibition of metabolism of these drugs by clarithromycin tablets. Fatalities have been reported.
4.3 Cholestatic Jaundice/Hepatic Dysfunction
Clarithromycin tablets are contraindicated in patients with a history of cholestatic jaundice or hepatic dysfunction associated with prior use of clarithromycin.
4.4 Colchicine
Concomitant administration of clarithromycin tablets and colchicine is contraindicated in patients with renal or hepatic impairment.
4.5 Lomitapide, Lovastatin, and Simvastatin
Concomitant administration of clarithromycin tablets with lomitapide is contraindicated due to potential for markedly increased transaminases [see Warnings and Precautions (5.4) and Drug Interactions (7)].
Concomitant administration of clarithromycin tablets with HMG-CoA reductase inhibitors (statins) that are extensively metabolized by CYP3A4 (lovastatin or simvastatin) is contraindicated, due to the increased risk of myopathy, including rhabdomyolysis [see Warnings and Precautions (5.4) and Drug Interactions (7)].
4.6 Ergot Alkaloids
Concomitant administration of clarithromycin and ergotamine or dihydroergotamine is contraindicated [see Drug Interactions (7)].
4.7 Lurasidone
Concomitant administration of clarithromycin and lurasidone is contraindicated since it may result in an increase in lurasidone exposure and the potential for serious adverse reactions [see Drug Interactions (7)].
Close4.8 Contraindications for Co-administered Drugs
For information about contraindications of other drugs indicated in combination with clarithromycin tablets, refer to their full prescribing information (contraindications section).
-
5 WARNINGS AND PRECAUTIONS5.1 Severe Acute Hypersensitivity Reactions - In the event of severe acute hypersensitivity reactions, such as anaphylaxis, Stevens-Johnson syndrome, toxic epidermal necrolysis, drug rash with ...
5.1 Severe Acute Hypersensitivity Reactions
In the event of severe acute hypersensitivity reactions, such as anaphylaxis, Stevens-Johnson syndrome, toxic epidermal necrolysis, drug rash with eosinophilia and systemic symptoms (DRESS), Henoch-Schonlein purpura, and acute generalized exanthematous pustulosis, discontinue clarithromycin therapy immediately and institute appropriate treatment.
5.2 QT Prolongation
- •
- Clarithromycin has been associated with prolongation of the QT interval and infrequent cases of arrhythmia. Cases of torsades de pointes have been spontaneously reported during postmarketing surveillance in patients receiving clarithromycin. Fatalities have been reported.
Avoid clarithromycin in the following patients:
- •
- patients with known prolongation of the QT interval, ventricular cardiac arrhythmia, including torsades de pointes
- •
- patients receiving drugs known to prolong the QT interval [see also Contraindications (4.2)]
- •
- patients with ongoing proarrhythmic conditions such as uncorrected hypokalemia or hypomagnesemia, clinically significant bradycardia and in patients receiving Class IA (e.g., quinidine, procainamide, disopyramide) or Class III (e.g., dofetilide, amiodarone, sotalol) antiarrhythmic agents.
Elderly patients may be more susceptible to drug-associated effects on the QT interval [see Use in Specific Populations (8.5)].
5.3 Hepatotoxicity
Hepatic dysfunction, including increased liver enzymes, and hepatocellular and/or cholestatic hepatitis, with or without jaundice, has been reported with clarithromycin. This hepatic dysfunction may be severe and is usually reversible. In some instances, hepatic failure with fatal outcome has been reported and generally has been associated with serious underlying diseases and/or concomitant medications. Symptoms of hepatitis can include anorexia, jaundice, dark urine, pruritus, or tender abdomen. Discontinue clarithromycin immediately if signs and symptoms of hepatitis occur.
5.4 Serious Adverse Reactions Due to Concomitant Use with Other Drugs
Drugs metabolized by CYP3A4: Serious adverse reactions have been reported in patients taking clarithromycin concomitantly with CYP3A4 substrates. These include colchicine toxicity with colchicine; markedly increased transaminases with lomitapide; rhabdomyolysis with simvastatin, lovastatin, and atorvastatin; hypoglycemia and cardiac arrhythmias (e.g., torsades de pointes) with disopyramide; and hypotension and acute kidney injury with calcium channel blockers metabolized by CYP3A4 (e.g., verapamil, amlodipine, diltiazem, nifedipine). Most reports of acute kidney injury with calcium channel blockers metabolized by CYP3A4 involved elderly patients 65 years of age or older. Use clarithromycin with caution when administered concurrently with medications that induce the cytochrome CYP3A4 enzyme. The use of clarithromycin with lomitapide, simvastatin, lovastatin, ergotamine, or dihydroergotamine is contraindicated [see Contraindications (4.5, 4.6) and Drug Interactions (7)].
Colchicine: Life-threatening and fatal drug interactions have been reported in patients treated with clarithromycin and colchicine. Clarithromycin is a strong CYP3A4 inhibitor and this interaction may occur while using both drugs at their recommended doses. If co-administration of clarithromycin and colchicine is necessary in patients with normal renal and hepatic function, reduce the dose of colchicine. Monitor patients for clinical symptoms of colchicine toxicity. Concomitant administration of clarithromycin and colchicine is contraindicated in patients with renal or hepatic impairment [see Contraindications (4.4) and Drug Interactions (7)].
Lomitapide: Concomitant use of clarithromycin with lomitapide is contraindicated [see Contraindications (4.5)]. Lomitapide is metabolized by CYP3A4, and concomitant treatment with clarithromycin increases the plasma concentration of lomitapide, which increases the risk of elevation in transaminases [see Drug Interactions (7)]. If treatment with clarithromycin cannot be avoided, therapy with lomitapide must be suspended during the course of treatment.
HMG-CoA Reductase Inhibitors (statins): Concomitant use of clarithromycin with lovastatin or simvastatin is contraindicated [see Contraindications (4.5)] as these statins are extensively metabolized by CYP3A4, and concomitant treatment with clarithromycin increases their plasma concentration, which increases the risk of myopathy, including rhabdomyolysis. Cases of rhabdomyolysis have been reported in patients taking clarithromycin concomitantly with these statins. If treatment with clarithromycin cannot be avoided, therapy with lovastatin or simvastatin must be suspended during the course of treatment.
Exercise caution when prescribing clarithromycin with atorvastatin or pravastatin. In situations where the concomitant use of clarithromycin with atorvastatin or pravastatin cannot be avoided, atorvastatin dose should not exceed 20 mg daily and pravastatin dose should not exceed 40 mg daily. Use of a statin that is not dependent on CYP3A metabolism (e.g., fluvastatin) can be considered. It is recommended to prescribe the lowest registered dose if concomitant use cannot be avoided.
Oral Hypoglycemic Agents/Insulin: The concomitant use of clarithromycin and oral hypoglycemic agents and/or insulin can result in significant hypoglycemia. With certain hypoglycemic drugs such as nateglinide, pioglitazone, repaglinide and rosiglitazone, inhibition of CYP3A enzyme by clarithromycin may be involved and could cause hypoglycemia when used concomitantly. Careful monitoring of glucose is recommended [see Drug Interactions (7)].
Quetiapine: Use quetiapine and clarithromycin concomitantly with caution. Co-administration could result in increased quetiapine exposure and quetiapine related toxicities such as somnolence, orthostatic hypotension, altered state of consciousness, neuroleptic malignant syndrome, and QT prolongation. Refer to quetiapine prescribing information for recommendations on dose reduction if co-administered with CYP3A4 inhibitors such as clarithromycin [see Drug Interactions (7)].
Oral Anticoagulants: There is a risk of serious hemorrhage and significant elevations in INR and prothrombin time when clarithromycin is co-administered with warfarin. Monitor INR and prothrombin times frequently while patients are receiving clarithromycin and oral anticoagulants concurrently [see Drug Interactions (7)].
Benzodiazepines: Increased sedation and prolongation of sedation have been reported with concomitant administration of clarithromycin and triazolobenzodiazepines, such as triazolam and midazolam [see Drug Interactions (7)].
5.5 All-Cause Mortality in Patients With Coronary Artery Disease 1 to 10 Years After Clarithromycin Exposure
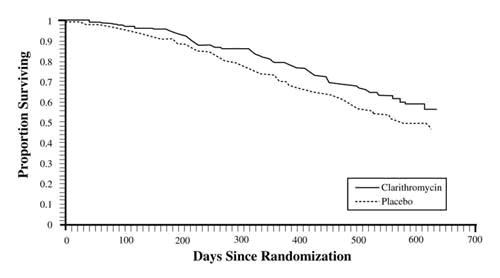
In one clinical trial evaluating treatment with clarithromycin on outcomes in patients with coronary artery disease, an increase in risk of all-cause mortality one year or more after the end of treatment was observed in patients randomized to receive clarithromycin.1 Clarithromycin for treatment of coronary artery disease is not an approved indication. The cause of the increased risk has not been established. Other epidemiologic studies evaluating this risk have shown variable results [see Adverse Reactions (6.1)]. Consider balancing this potential risk with the treatment benefits when prescribing clarithromycin in patients who have suspected or confirmed coronary artery disease.
5.6 Clostridium difficile Associated Diarrhea
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including clarithromycin, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.7 Embryo-Fetal Toxicity
Based on findings from animal studies, clarithromycin is not recommended for use in pregnant women except in clinical circumstances where no alternative therapy is appropriate. If clarithromycin is used during pregnancy, or if pregnancy occurs while the patient is taking this drug, the patient should be apprised of the potential hazard to the fetus. Clarithromycin demonstrated adverse effects on pregnancy outcome and/or embryo fetal development, including fetal malformations, in pregnant animals administered oral clarithromycin [see Use in Specific Populations (8.1)].
5.8 Exacerbation of Myasthenia Gravis
Exacerbation of symptoms of myasthenia gravis and new onset of symptoms of myasthenic syndrome has been reported in patients receiving clarithromycin therapy.
Close5.9 Development of Drug Resistant Bacteria
Prescribing clarithromycin in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described below and elsewhere in the labeling: • Acute Hypersensitivity Reactions [see Warnings and Precautions (5.1)] • QT Prolongation [see ...
The following serious adverse reactions are described below and elsewhere in the labeling:
• Acute Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
• QT Prolongation [see Warnings and Precautions (5.2)]
• Hepatotoxicity [see Warnings and Precautions (5.3)]
• Serious Adverse Reactions Due to Concomitant Use with Other Drugs [see Warnings and Precautions (5.4)]
• Clostridium difficile Associated Diarrhea [see Warnings and Precautions (5.6)]
• Exacerbation of Myasthenia Gravis [see Warnings and Precautions (5.8)]6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Based on pooled data across all indications, the most frequent adverse reactions for both adult and pediatric populations observed in clinical trials are abdominal pain, diarrhea, nausea, vomiting and dysgeusia. Also reported were dyspepsia, liver function test abnormal, anaphylactic reaction, candidiasis, headache, insomnia, and rash.
The subsequent subsections list the most common adverse reactions for prophylaxis and treatment of mycobacterial infections and duodenal ulcer associated with H. pylori infection. In general, these profiles are consistent with the pooled data described above.
Prophylaxis of Mycobacterial Infections
In AIDS patients treated with clarithromycin over long periods of time for prophylaxis against M. avium, it was often difficult to distinguish adverse reactions possibly associated with clarithromycin administration from underlying HIV disease or intercurrent illness. Median duration of treatment was 10.6 months for the clarithromycin group and 8.2 months for the placebo group.
Table 4. Incidence Rates (%) of Selected Adverse Reactionsa in Immunocompromised Adult Patients Receiving Prophylaxis Against M. avium Complex
Body Systemb
Adverse Reaction
Clarithromycin
(n=339)
%
Placebo
(n=339)
%
Body as a Whole
Abdominal pain
5%
4%
Headache
3%
1%
Digestive
Diarrhea
8%
4%
Dyspepsia
4%
3%
Flatulence
2%
1%
Nausea
11%
7%
Vomiting
6%
3%
Skin & Appendages
Rash
3%
4%
Special Senses
Taste Perversion
8%c
0.3%
a Includes those events possibly or probably related to study drug and excludes concurrent conditions
b 2% or greater Adverse Reaction Incidence Rates for either treatment group
c Significant higher incidence compared to the placebo-treated group
Discontinuation due to adverse reactions occurred in 18% of patients receiving clarithromycin compared to 17% of patients receiving placebo in this trial. Primary reasons for discontinuation in clarithromycin treated patients include headache, nausea, vomiting, depression, and taste perversion.
Changes in Laboratory Values
Selected laboratory adverse experiences that were reported during therapy in greater than 2 % of adult patients treated with clarithromycin in a randomized double-blind clinical trial involving 682 patients are presented in Table 5.
In immunocompromised patients receiving prophylaxis against M. avium, evaluations of laboratory values were made by analyzing those values outside the seriously abnormal value (i.e., the extreme high or low limit) for the specified test.
Table 5. Percentage of Patientsa Exceeding Extreme Laboratory Values in Patients Receiving Prophylaxis Against M. avium Complex
Clarithromycin 500 mg
twice a day
Placebo
WBC Count
<1 x 109/L
2/103 (4%)
0/95
SGOT
>5 x ULNb
7/196 (4%)
5/208 (2%)
SGPT
>5 x ULNb
6/217 (3%)
4/232 (2%)
a Includes only patients with baseline values within the normal range or borderline high (hematology variables) and within normal range or borderline low (chemistry variables)
b ULN= Upper Limit of Normal
Treatment of Mycobacterial Infections
The adverse reaction profiles for both the 500 mg and 1000 mg twice a day dose regimens were similar.
In AIDS patients and other immunocompromised patients treated with the higher doses of clarithromycin over long periods of time for mycobacterial infections, it was often difficult to distinguish adverse reactions possibly associated with clarithromycin administration from underlying signs of HIV disease or intercurrent illness.
The following analysis summarizes experience during the first 12 weeks of therapy with clarithromycin. Data are reported separately for trial 1 (randomized, double-blind) and trial 2 (open-labeled, compassionate use) and also combined. Adverse reactions were reported less frequently in trial 2, which may be due in part to differences in monitoring between the two studies.
In adult patients receiving clarithromycin 500 mg twice a day, the most frequently reported adverse reactions, considered possibly or possibly related to study drug, with an incidence of 5% or greater, are listed below (Table 6). Approximately 8% of the patients who received 500 mg twice a day and 12% of the patients who received 1000 mg twice a day discontinued therapy due to drug related adverse reactions during the first 12 weeks of therapy; adverse reactions leading to discontinuation in at least 2 patients included nausea, vomiting, abdominal pain, diarrhea, rash, and asthenia.
Table 6. Selected Treatment-Relateda Adverse Reaction Incidence Rates (%) in Immunocompromised Adult Patients During the First 12 Weeks of Therapy with 500 mg Twice a Day Clarithromycin Dose
Adverse Reaction
Trial 1
(n=53)
Trial 2
(n=255)
Combined
(n=308)
Abdominal Pain
8
2
3
Diarrhea
9
2
3
Flatulence
8
0
1
Headache
8
0
2
Nausea
28
9
12
Rash
9
2
3
Taste Perversion
19
0
4
Vomiting
25
4
8
a Includes those events possibly or probably related to study drug and excludes concurrent conditions
A limited number of pediatric AIDS patients have been treated with clarithromycin suspension for mycobacterial infections. The most frequently reported adverse reactions excluding those due to the patient’s concurrent conditions were consistent with those observed in adult patients.
Changes in Laboratory Values
In the first 12 weeks of starting on clarithromycin 500 mg twice a day, 3% of patients has SGOT increases and 2% of patients has SGPT increases > 5 times the upper limit of normal in trial 2 (469 enrolled adult patients) while trial 1 (154 enrolled patients) had no elevation of transaminases. This includes only patients with baseline values within the normal range or borderline low.
Duodenal ulcer associated with H. pylori Infection
In clinical trials using combination therapy with clarithromycin plus omeprazole and amoxicillin, no adverse reactions specific to the combination of these drugs have been observed. Adverse reactions that have occurred have been limited to those that have been previously reported with clarithromycin, omeprazole or amoxicillin.
The adverse reaction profiles are shown below (Table 7) for four randomized double-blind clinical trials in which patients received the combination of clarithromycin 500 mg three times a day, and omeprazole 40 mg daily for 14 days, followed by omeprazole 20 mg once a day, (three studies) or 40 mg once a day (one study) for an additional 14 days. Of the 346 patients who received the combination, 3.5% of patients discontinued drug due to adverse reactions.
Table 7. Adverse Reactions with an Incidence of 3% or Greater
Adverse Reaction
Clarithromycin + Omeprazole
(n=346)
% of Patients
Omeprazole
(n=355)
% of Patients
Clarithromycin (n=166)
% of Patientsa
Taste Perversion
15
1
16
Nausea
5
1
3
Headache
5
6
9
Diarrhea
4
3
7
Vomiting
4
<1
1
Abdominal Pain
3
2
1
Infection
3
4
2
a Only two of four studies
Changes in Laboratory Values
Changes in laboratory values with possible clinical significance in patients taking clarithromycin and omeprazole in four randomized double-blind trials in 945 patients are as follows:
Hepatic: elevated direct bilirubin <1%; GGT <1%; SGOT (AST) <1%; SGPT (ALT) <1%, Renal: elevated serum creatinine <1%.
Less Frequent Adverse Reactions Observed During Clinical Trials of Clarithromycin
Based on pooled data across all indications, the following adverse reactions were observed in clinical trials with clarithromycin at a rate less than 1%:
Blood and Lymphatic System Disorders: Leukopenia, neutropenia, thrombocythemia, eosinophilia
Cardiac Disorders: Electrocardiogram QT prolonged, cardiac arrest, atrial fibrillation, extrasystoles, palpitations
Ear and Labyrinth Disorders: Vertigo, tinnitus, hearing impaired
Gastrointestinal Disorders: Stomatitis, glossitis, esophagitis, gastrooesophageal reflux disease, gastritis, proctalgia, abdominal distension, constipation, dry mouth, eructation, flatulence
General Disorders and Administration Site Conditions: Malaise, pyrexia, asthenia, chest pain, chills, fatigue
Hepatobiliary Disorders: Cholestasis, hepatitis
Immune System Disorders: Hypersensitivity
Infections and Infestations: Cellulitis, gastroenteritis, infection, vaginal infection
Investigations: Blood bilirubin increased, blood alkaline phosphatase increased, blood lactate
dehydrogenase increased, albumin globulin ratio abnormal
Metabolism and Nutrition Disorders: Anorexia, decreased appetite
Musculoskeletal and Connective Tissue Disorders: Myalgia, muscle spasms, nuchal rigidity
Nervous System Disorders: Dizziness, tremor, loss of consciousness, dyskinesia, somnolence
Psychiatric Disorders: Anxiety, nervousness
Renal and Urinary Disorders: Blood creatinine increased, blood urea increased
Respiratory, Thoracic and Mediastinal Disorders: Asthma, epistaxis, pulmonary embolism
Skin and Subcutaneous Tissue Disorders: Urticaria, dermatitis bullous, pruritus, hyperhidrosis, rash maculo-papular
Gastrointestinal Adverse Reactions
In the acute exacerbation of chronic bronchitis and acute maxillary sinusitis studies overall gastrointestinal adverse reactions were reported by a similar proportion of patients taking either clarithromycin tablets or clarithromycin extended-release tablets; however, patients taking clarithromycin extended-release tablets reported significantly less severe gastrointestinal symptoms compared to patients taking clarithromycin tablets. In addition, patients taking clarithromycin extended-release tablets had significantly fewer premature discontinuations for drug-related gastrointestinal or abnormal taste adverse reactions compared to clarithromycin tablets.
All-Cause Mortality in Patients with Coronary Artery Disease 1 to 10 Years Following Clarithromycin Exposure
In one clinical trial evaluating treatment with clarithromycin on outcomes in patients with coronary artery disease, an increase in risk of all-cause mortality was observed in patients randomized to clarithromycin. Clarithromycin for treatment of coronary artery disease is not an approved indication. Patients were treated with clarithromycin or placebo for 14 days and observed for primary outcome events (e.g., all-cause mortality or non-fatal cardiac events) for several years.1 A numerically higher number of primary outcome events in patients randomized to receive clarithromycin was observed with a hazard ratio of 1.06 (95% confidence interval 0.98 to 1.14). However, at follow-up 10 years post-treatment, there were 866 (40%) deaths in the clarithromycin group and 815 (37%) deaths in the placebo group that represented a hazard ratio for all-cause mortality of 1.10 (95% confidence interval 1.00 to 1.21). The difference in the number of deaths emerged after one year or more after the end of treatment.
The cause of the difference in all-cause mortality has not been established. Other epidemiologic studies evaluating this risk have shown variable results [see Warnings and Precautions (5.5)].
Close6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of clarithromycin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System: Thrombocytopenia, agranulocytosis
Cardiac: Ventricular arrhythmia, ventricular tachycardia, torsades de pointes
Ear and Labyrinth: Deafness was reported chiefly in elderly women and was usually reversible.
Gastrointestinal: Pancreatitis acute, tongue discoloration, tooth discoloration was reported and was usually reversible with professional cleaning upon discontinuation of the drug.
There have been reports of clarithromycin extended-release tablets in the stool, many of which have occurred in patients with anatomic (including ileostomy or colostomy) or functional gastrointestinal disorders with shortened GI transit times. In several reports, tablet residues have occurred in the context of diarrhea. It is recommended that patients who experience tablet residue in the stool and no improvement in their condition should be switched to a different clarithromycin formulation (e.g., suspension) or another antibacterial drug.
Hepatobiliary: Hepatic failure, jaundice hepatocellular. Adverse reactions related to hepatic dysfunction have been reported with clarithromycin [see Warnings and Precautions (5.2)].Infections and Infestations: Pseudomembranous colitis [see Warnings and Precautions (5.6)]
Immune System: Anaphylactic reactions, angioedema
Investigations: Prothrombin time prolonged, white blood cell count decreased, international normalized ratio increased. Abnormal urine color has been reported, associated with hepatic failure.
Metabolism and Nutrition: Hypoglycemia has been reported in patients taking oral hypoglycemic agents or insulin.
Musculoskeletal and Connective Tissue: Myopathy rhabdomyolysis was reported and in some of the reports, clarithromycin was administered concomitantly with statins, fibrates, colchicine or allopurinol [see Contraindications (4.5) and Warnings and Precautions (5.4)].
Nervous System: Parosmia, anosmia, ageusia, paresthesia and convulsions
Psychiatric: Abnormal behavior, confusional state, depersonalization, disorientation, hallucination, depression, manic behavior, abnormal dream, psychotic disorder. These disorders usually resolve upon discontinuation of the drug.
Renal and Urinary: Nephritis interstitial, renal failure
Skin and Subcutaneous Tissue: Stevens-Johnson syndrome, toxic epidermal necrolysis, drug rash with eosinophilia and systemic symptoms (DRESS), Henoch-Schonlein purpura, acne, acute generalized exanthematous pustulosis
Vascular: Hemorrhage
-
7 DRUG INTERACTIONSCo-administration of clarithromycin is known to inhibit CYP3A, and a drug primarily metabolized by CYP3A may be associated with elevations in drug concentrations that could increase or prolong ...
Co-administration of clarithromycin is known to inhibit CYP3A, and a drug primarily metabolized by CYP3A may be associated with elevations in drug concentrations that could increase or prolong both therapeutic and adverse effects of the concomitant drug.
Clarithromycin should be used with caution in patients receiving treatment with other drugs known to be CYP3A enzyme substrates, especially if the CYP3A substrate has a narrow safety margin (e.g., carbamazepine) and/or the substrate is extensively metabolized by this enzyme. Adjust dosage when appropriate and monitor serum concentrations of drugs primarily metabolized by CYP3A closely in patients concurrently receiving clarithromycin.
Table 8: Clinically Significant Drug Interactions with Clarithromycin
CloseDrugs That Are Affected By Clarithromycin
Drug(s) with Pharmacokinetics Affected by Clarithromycin
Recommendation
Comments
Antiarrhythmics:
Disopyramide
Quinidine
Dofetilide
Amiodarone
Sotalol
Procainamide
Digoxin
Not Recommended
Use With Caution
Disopyramide, Quinidine: There have been postmarketing reports of torsades de pointes occurring with concurrent use of clarithromycin and quinidine or disopyramide. Electrocardiograms should be monitored for QTc prolongation during coadministration of clarithromycin with these drugs [see Warnings and Precautions (5.2)].
Serum concentrations of these medications should also be monitored. There have been spontaneous or published reports of CYP3A based interactions of clarithromycin with disopyramide and quinidine.
There have been postmarketing reports of hypoglycemia with the concomitant administration of clarithromycin and disopyramide. Therefore, blood glucose levels should be monitored during concomitant administration of clarithromycin and disopyramide.
Digoxin: Digoxin is a substrate for P-glycoprotein (Pgp) and clarithromycin is known to inhibit Pgp. When clarithromycin and digoxin are co-administered, inhibition of Pgp by clarithromycin may lead to increased exposure of digoxin. Elevated digoxin serum concentrations in patients receiving clarithromycin and digoxin concomitantly have been reported in postmarketing surveillance. Some patients have shown clinical signs consistent with digoxin toxicity, including potentially fatal arrhythmias. Monitoring of serum digoxin concentrations should be considered, especially for patients with digoxin concentrations in the upper therapeutic range.
Oral Anticoagulants:
Warfarin
Use With Caution
Oral anticoagulants: Spontaneous reports in the postmarketing period suggest that concomitant administration of clarithromycin and oral anticoagulants may potentiate the effects of the oral anticoagulants. Prothrombin times should be carefully monitored while patients are receiving clarithromycin and oral anticoagulants simultaneously [see Warnings and Precautions (5.4)].
Antiepileptics:
Carbamazepine
Use With Caution
Carbamazepine: Concomitant administration of single doses of clarithromycin and carbamazepine has been shown to result in increased plasma concentrations of carbamazepine. Blood level monitoring of carbamazepine may be considered. Increased serum concentrations of carbamazepine were observed in clinical trials with clarithromycin. There have been spontaneous or published reports of CYP3A based interactions of clarithromycin with carbamazepine.
Antifungals:
Itraconazole
Fluconazole
Use With Caution
No Dose Adjustment
Itraconazole: Both clarithromycin and itraconazole are substrates and inhibitors of CYP3A, potentially leading to a bi-directional drug interaction when administered concomitantly (see also Itraconazole under “Drugs That Affect Clarithromycin” in the table below). Clarithromycin may increase the plasma concentrations of itraconazole. Patients taking itraconazole and clarithromycin concomitantly should be monitored closely for signs or symptoms of increased or prolonged adverse reactions.
Fluconazole: [see Pharmacokinetics (12.3)]
Anti-Gout Agents:
Colchicine (in patients with renal or hepatic impairment)
Colchicine (in patients with normal renal and hepatic function)
Contraindicated
Use With Caution
Colchicine: Colchicine is a substrate for both CYP3A and the efflux transporter, P-glycoprotein (Pgp). Clarithromycin and other macrolides are known to inhibit CYP3A and Pgp. The dose of colchicine should be reduced when co-administered with clarithromycin in patients with normal renal and hepatic function [see Contraindications (4.4) and Warnings and Precautions (5.4)].
Antipsychotics:
Pimozide
Quetiapine
Lurasidone
Contraindicated
Pimozide: [See Contraindications (4.2)]
Quetiapine: Quetiapine is a substrate for CYP3A4, which is inhibited by clarithromycin. Co-administration with clarithromycin could result in increased quetiapine exposure and possible quetiapine related toxicities. There have been postmarketing reports of somnolence, orthostatic hypotension, altered state of consciousness, neuroleptic malignant syndrome, and QT prolongation during concomitant administration. Refer to quetiapine prescribing information for recommendations on dose reduction if co-administered with CYP3A4 inhibitors such as clarithromycin.
Lurasidone: [See Contraindications (4.7)]
Antispasmodics:
Tolterodine (patients deficient in CYP2D6 activity)
Use With Caution
Tolterodine: The primary route of metabolism for tolterodine is via. CYP2D6. However, in a subset of the population devoid of CYP2D6, the identified pathway of metabolism is via. CYP3A. In this population subset, inhibition of CYP3A results in significantly higher serum concentrations of tolterodine. Tolterodine 1 mg twice daily is recommended in patients deficient in CYP2D6 activity (poor metabolizers) when co-administered with clarithromycin.
Antivirals:
Atazanavir
Saquinavir (in patients with decreased renal function)
Ritonavir
Etravirine
Maraviroc
Boceprevir (in patients with normal renal function)
Didanosine
Zidovudine
Use With Caution
No Dose Adjustment
Atazanavir: Both clarithromycin and atazanavir are substrates and inhibitors of CYP3A, and there is evidence of a bi-directional drug interaction (see Atazanavir under “Drugs That Affect Clarithromycin” in the table below) [see Pharmacokinetics (12.3)].
Saquinavir: Both clarithromycin and saquinavir are substrates and inhibitors of CYP3A and there is evidence of a bi-directional drug interaction (see Saquinavir under “Drugs That Affect Clarithromycin” in the table below) [see Pharmacokinetics (12.3)].
Ritonavir, Etravirine: (see Ritonavir and Etravirine under “Drugs That Affect Clarithromycin” in the table below) [see Pharmacokinetics (12.3)].
Maraviroc: Clarithromycin may result in increases in maraviroc exposures by inhibition of CYP3A metabolism. See Selzentry® prescribing information for dose recommendation when given with strong CYP3A inhibitors such as clarithromycin.
Boceprevir: Both clarithromycin and boceprevir are substrates and inhibitors of CYP3A, potentially leading to a bi-directional drug interaction when co-administered. No dose adjustments are necessary for patients with normal renal function (see Victrelis® prescribing information).
Zidovudine: Simultaneous oral administration of clarithromycin immediate-release tablets and zidovudine to HIV-infected adult patients may result in decreased steady-state zidovudine concentrations. Administration of clarithromycin and zidovudine should be separated by at least two hours [see Pharmacokinetics (12.3)].
Calcium Channel Blockers:
Verapamil
Amlodipine
Diltiazem
Nifedipine
Use With Caution
Verapamil: Hypotension, bradyarrhythmias, and lactic acidosis have been observed in patients receiving concurrent verapamil, [see Warnings and Precautions (5.4)].
Amlodipine, Diltiazem: [See Warnings and Precautions (5.4)]
Nifedipine: Nifedipine is a substrate for CYP3A. Clarithromycin and other macrolides are known to inhibit CYP3A. There is potential of CYP3A-mediated interaction between nifedipine and clarithromycin. Hypotension and peripheral edema were observed when clarithromycin was taken concomitantly with nifedipine [see Warnings and Precautions (5.4)].
Ergot Alkaloids:
Ergotamine
Dihydroergotamine
Contraindicated
Ergotamine, Dihydroergotamine: Postmarketing reports indicate that coadministration of clarithromycin with ergotamine or dihydroergotamine has been associated with acute ergot toxicity characterized by vasospasm and ischemia of the extremities and other tissues including the central nervous system [see Contraindications (4.6)].
Gastroprokinetic Agents:
Cisapride
Contraindicated
Cisapride: [See Contraindications (4.2)]
Lipid-lowering agents:
Lomitapide
Lovastatin
Simvastatin
Atorvastatin
Pravastatin
Fluvastatin
Contraindicated
Use With Caution
No Dose Adjustment
Lomitapide, Lovastatin, Simvastatin: Clarithromycin may increase the exposure of these drugs by inhibition of CYP3A metabolism, thereby increasing the risk of toxicities from these drugs [see Contraindications (4.5) and Warnings and Precautions (5.4)]
Atorvastatin, Pravastatin, Fluvastatin: [See Warnings and Precautions (5.4)]Hypoglycemic Agents:
Nateglinide
Pioglitazone
Repaglinide
Rosiglitazone
Insulin
Use With Caution
Nateglinide, Pioglitazone, Repaglinide, Rosiglitazone: [See Warnings and Precautions (5.4) and Adverse Reactions (6.2)]
Insulin: [See Warnings and Precautions (5.4) and Adverse Reactions (6.2)]
Immunosuppressants:
Cyclosporine
Tacrolimus
Use With Caution
Cyclosporine: There have been spontaneous or published reports of CYP3A based interactions of clarithromycin with cyclosporine.
Tacrolimus: There have been spontaneous or published reports of CYP3A based interactions of clarithromycin with tacrolimus.
Phosphodiesterase inhibitors:
Sildenafil
Tadalafil
Vardenafil
Use With Caution
Sildenafil, Tadalafil, Vardenafil: Each of these phosphodiesterase inhibitors is primarily metabolized by CYP3A, and CYP3A will be inhibited by concomitant administration of clarithromycin. Co-administration of clarithromycin with sildenafil, tadalafil, or vardenafil will result in increased exposure of these phosphodiesterase inhibitors. Co-administration of these phosphodiesterase inhibitors with clarithromycin is not recommended. Increased systemic exposure of these drugs may occur with clarithromycin; reduction of dosage for phosphodiesterase inhibitors should be considered (see their respective prescribing information).
Proton Pump Inhibitors:
Omeprazole
No Dose Adjustment
Omeprazole: The mean 24-hour gastric pH value was 5.2 when omeprazole was administered alone and 5.7 when coadministered with clarithromycin as a result of increased omeprazole exposures [see Pharmacokinetics (12.3)] (see also Omeprazole under “Drugs That Affect Clarithromycin” in the table below).
Xanthine Derivatives:
Theophylline
Use With Caution
Theophylline: Clarithromycin use in patients who are receiving theophylline may be associated with an increase of serum theophylline concentrations [see Pharmacokinetics (12.3)]. Monitoring of serum theophylline concentrations should be considered for patients receiving high doses of theophylline or with baseline concentrations in the upper therapeutic range.
Triazolobenzodiazepines and Other Related Benzodiazepines:
Midazolam
Alprazolam
Triazolam
Temazepam
Nitrazepam
Lorazepam
Use With Caution
No Dose Adjustment
Midazolam: When oral midazolam is co-administered with clarithromycin, dose adjustments may be necessary and possible prolongation and intensity of effect should be anticipated [see Warnings and Precautions (5.4) and Pharmacokinetics (12.3)].
Triazolam, Alprazolam: Caution and appropriate dose adjustments should be considered when triazolam or alprazolam is co-administered with clarithromycin. There have been postmarketing reports of drug interactions and central nervous system (CNS) effects (e.g., somnolence and confusion) with the concomitant use of clarithromycin and triazolam. Monitoring the patient for increased CNS pharmacological effects is suggested.
In postmarketing experience, erythromycin has been reported to decrease the clearance of triazolam and midazolam, and thus, may increase the pharmacologic effect of these benzodiazepines.
Temazepam, Nitrazepam, Lorazepam: For benzodiazepines which are not metabolized by CYP3A (e.g., temazepam, nitrazepam, lorazepam), a clinically important interaction with clarithromycin is unlikely.
Cytochrome P450 Inducers:
Rifabutin
Use With Caution
Rifabutin: Concomitant administration of rifabutin and clarithromycin resulted in an increase in rifabutin, and decrease in clarithromycin serum levels together with an increased risk of uveitis (see Rifabutin under “Drugs That Affect Clarithromycin” in the table below).
Other Drugs
Metabolized by CYP3A:
Alfentanil
Bromocriptine
Cilostazol
Methylprednisole Vinblastine
Phenobarbital
St. John’s Wort
Use With Caution
There have been spontaneous or published reports of CYP3A based interactions of clarithromycin with alfentanil, methylprednisolone, cilostazol, bromocriptine, vinblastine, phenobarbital, and St. John’s Wort.
Other Drugs Metabolized by CYP450 Isoforms Other than CYP3A:
Hexobarbital
Phenytoin
Valproate
Use With Caution
There have been postmarketing reports of interactions of clarithromycin with drugs not thought to be metabolized by CYP3A, including hexobarbital, phenytoin, and valproate.
Drugs that Affect Clarithromycin
Drug(s) that Affect the Pharmacokinetics of Clarithromycin
Recommendation
Comments
Antifungals:
Itraconazole
Use With Caution
Itraconazole: Itraconazole may increase the plasma concentrations of clarithromycin. Patients taking itraconazole and clarithromycin concomitantly should be monitored closely for signs or symptoms of increased or prolonged adverse reactions (see also Itraconazole under “Drugs That Are Affected By Clarithromycin” in the table above).
Antivirals:
Atazanavir
Ritonavir (in patients with decreased renal function)
Saquinavir (in patients with decreased renal function)
Etravirine
Saquinavir (in patients with normal renal function) Ritonavir (in patients with normal renal function)
Use With Caution
No Dose Adjustment
Atazanavir: When clarithromycin is co-administered with atazanavir, the dose of clarithromycin should be decreased by 50% [see Clinical Pharmacology (12.3)].
Since concentrations of 14-OH clarithromycin are significantly reduced when clarithromycin is co-administered with atazanavir, alternative antibacterial therapy should be considered for indications other than infections due to Mycobacterium avium complex. Doses of clarithromycin greater than 1000 mg per day should not be co-administered with protease inhibitors.
Ritonavir: Since concentrations of 14-OH clarithromycin are significantly reduced when clarithromycin is co-administered with ritonavir, alternative antibacterial therapy should be considered for indications other than infections due to Mycobacterium avium [see Pharmacokinetics (12.3)].
Doses of clarithromycin greater than 1000 mg per day should not be co-administered with protease inhibitors.
Saquinavir: When saquinavir is co-administered with ritonavir, consideration should be given to the potential effects of ritonavir on clarithromycin (refer to ritonavir above) [see Pharmacokinetics (12.3)].
Etravirine: Clarithromycin exposure was decreased by etravirine; however, concentrations of the active metabolite, 14-OH-clarithromycin, were increased. Because 14-OH-clarithromycin has reduced activity against Mycobacterium avium complex (MAC), overall activity against this pathogen may be altered; therefore alternatives to clarithromycin should be considered for the treatment of MAC.
Proton Pump Inhibitors:
Omeprazole
Use With Caution
Omeprazole: Clarithromycin concentrations in the gastric tissue and mucus were also increased by concomitant administration of omeprazole [see Pharmacokinetics (12.3)].
Miscellaneous Cytochrome P450 Inducers:
Efavirenz
Nevirapine
Rifampicin
Rifabutin
Rifapentine
Use With Caution
Inducers of CYP3A enzymes, such as efavirenz, nevirapine, rifampicin, rifabutin, and rifapentine will increase the metabolism of clarithromycin, thus decreasing plasma concentrations of clarithromycin, while increasing those of 14-OH-clarithromycin. Since the microbiological activities of clarithromycin and 14-OH-clarithromycin are different for different bacteria, the intended therapeutic effect could be impaired during concomitant administration of clarithromycin and enzyme inducers. Alternative antibacterial treatment should be considered when treating patients receiving inducers of CYP3A. There have been spontaneous or published reports of CYP3A based interactions of clarithromycin with rifabutin (see Rifabutin under “Drugs That Are Affected By Clarithromycin” in the table above).
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings from animal studies, clarithromycin is not recommended for use in pregnant women except in clinical circumstances where no alternative therapy ...
8.1 Pregnancy
Risk Summary
Based on findings from animal studies, clarithromycin is not recommended for use in pregnant women except in clinical circumstances where no alternative therapy is appropriate. If pregnancy occurs while taking clarithromycin, the patient should be apprised of the potential hazard to the fetus [see Warnings and Precautions (5.7)].
Limited data from a small number of published human studies with clarithromycin use during pregnancy are insufficient to inform drug-associated risks of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal reproduction studies, administration of oral clarithromycin to pregnant mice, rats, rabbits, and monkeys during the period of organogenesis produced malformations in rats (cardiovascular anomalies) and mice (cleft palate) at clinically relevant doses based on body surface area comparison. Fetal effects in mice, rats, and monkeys (e.g., reduced fetal survival, body weight, body weight gain) and implantation losses in rabbits were generally considered to be secondary to maternal toxicity (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Animal reproduction studies were conducted in mice, rats, rabbits, and monkeys with oral and intravenously administered clarithromycin. In pregnant mice, clarithromycin was administered during organogenesis (gestation day [GD] 6 to 15) at oral doses of 15, 60, 250, 500, or 1000 mg/kg/day. Reduced body weight observed in dams at 1000 mg/kg/day (3 times the maximum recommended human dose [MRHD] based on body surface area comparison) resulted in reduced survival and body weight of the fetuses. At ≥ 500 mg/kg/day, increases in the incidence of postimplantation loss and cleft palate in the fetuses were observed. No adverse developmental effects were observed in mice at ≤ 250 mg/kg/day (≤ 1 times MRHD based on body surface area comparison).
In pregnant Sprague Dawley rats, clarithromycin was administered during organogenesis (GD 6 to 15) at oral doses of 15, 50, or 150 mg/kg/day. Reductions in body weight and food consumption was observed in dams at 150 mg/kg/day. Increased resorptions and reduced body weight of the fetuses at this dose were considered secondary to maternal toxicity. Additionally, at 150 mg/kg/day (1 times MRHD based on body surface area comparison), a low incidence of cardiovascular anomalies (complete situs inversus, undivided truncus, IV septal defect) was observed in the fetuses. Clarithromycin did not cause adverse developmental effects in rats at 50 mg/kg/day (0.3 times MRHD based on body surface area comparison). Intravenous dosing of clarithromycin during organogenesis in rats (GD 6 to 15) at 15, 50, or 160 mg/kg/day was associated with maternal toxicity (reduced body weight, body-weight gain, and food consumption) at 160 mg/kg/day but no evidence of adverse developmental effects at any dose (≤ 1 times MRHD based on body surface area comparison).
In pregnant Wistar rat, clarithromycin was administered during organogenesis (GD 7 to 17) at oral doses of 10, 40, or 160 mg/kg/day. Reduced body weight and food consumption were observed in dams at 160 mg/kg/day but there was no evidence of adverse developmental effects at any dose (≤ 1 times MRHD based on body surface area comparison).
In pregnant rabbits, clarithromycin administered during organogenesis (GD 6 to 18) at oral doses of 10, 35, or 125 mg/kg/day resulted in reduced maternal food consumption and decreased body weight at the highest dose, with no evidence of any adverse developmental effects at any dose (≤ 2 times MRHD based on body surface area comparison). Intravenously administered clarithromycin to pregnant rabbits during organogenesis (GD 6 to 18) in rabbits at 20, 40, 80, or 160 mg/kg/day (≥ 0.3 times MRHD based on body surface area comparison) resulted in maternal toxicity and implantation losses at all doses.
In pregnant monkeys, clarithromycin was administered (GD 20 to 50) at oral doses of 35 or 70 mg/kg/day. Dose-dependent emesis, poor appetite, fecal changes, and reduced body weight were observed in dams at all doses (≥ 0.5 times MRHD based on body surface area comparison). Growth retardation in 1 fetus at 70 mg/kg/day was considered secondary to maternal toxicity. There was no evidence of primary drug related adverse developmental effects at any dose tested.
In a reproductive toxicology study in rats administered oral clarithromycin late in gestation through lactation (GD 17 to post-natal day 21) at doses of 10, 40, or 160 mg/kg/day (≤ 1 times MRHD based on body surface area comparison), reductions in maternal body weight and food consumption were observed at 160 mg/kg/day. Reduced body-weight gain observed in offspring at 160 mg/kg/day was considered secondary to maternal toxicity. No adverse developmental effects were observed with clarithromycin at any dose tested.8.2 Lactation
Risk Summary
Based on limited human data, clarithromycin and its active metabolite 14-OH clarithromycin are present in human milk at less than 2% of the maternal weight-adjusted dose (see Data). In a separate observational study, reported adverse effects on breast-fed children (rash, diarrhea, loss of appetite, somnolence) were comparable to amoxicillin (see Data). No data are available to assess the effects of clarithromycin or 14-OH clarithromycin on milk production.
The development and health benefits of breastfeeding should be considered along with the mother’s clinical need for clarithromycin and any potential adverse effects on the breast-fed child from clarithromycin or from the underlying maternal condition.
Data
Human
Serum and milk samples were obtained after 3 days of treatment, at steady state, from one published study of 12 lactating women who were taking clarithromycin 250 mg orally twice daily. Based on the limited data from this study, and assuming milk consumption of 150 mL/kg/day, an exclusively human milk fed infant would receive an estimated average of 136 mcg/kg/day of clarithromycin and its active metabolite, with this maternal dosage regimen. This is less than 2% of the maternal weight-adjusted dose (7.8 mg/kg/day, based on the average maternal weight of 64 kg), and less than 1% of the pediatric dose (15 mg/kg/day) for children greater than 6 months of age.
A prospective observational study of 55 breastfed infants of mothers taking a macrolide antibacterial (6 were exposed to clarithromycin) were compared to 36 breastfed infants of mothers taking amoxicillin. Adverse reactions were comparable in both groups. Adverse reactions occurred in 12.7% of infants exposed to macrolides and included rash, diarrhea, loss of appetite, and somnolence.8.3 Females and Males of Reproductive Potential
Males
Administration of clarithromycin resulted in testicular atrophy in rats, dogs and monkeys [see Nonclinical Toxicology (13.1)].8.4 Pediatric Use
The safety and effectiveness of clarithromycin tablets have been established for the treatment of the following conditions or diseases in pediatric patients 6 months and older. Use in these indications is based on clinical trials in pediatric patients or adequate and well-controlled studies in adults with additional pharmacokinetic and safety data in pediatric patients:
- •
- Pharyngitis/Tonsillitis
- •
- Community-Acquired Pneumonia
- •
- Acute maxillary sinusitis
- •
- Acute otitis media [see Clinical Studies (14.2)]
- •
- Uncomplicated skin and skin structure infections
The safety and effectiveness of clarithromycin tablets have been established for the prevention of disseminated Mycobacterium avium complex (MAC) disease in pediatric patients 20 months and older with advanced HIV infection. No studies of clarithromycin for MAC prophylaxis have been performed in pediatric populations and the doses recommended for prophylaxis are derived from MAC pediatric treatment studies.
Safety and effectiveness of clarithromycin in pediatric patients under 6 months of age have not been established. The safety of clarithromycin has not been studied in MAC patients under the age of 20 months.
8.5 Geriatric Use
In a steady-state study in which healthy elderly subjects (65 years to 81 years of age) were given 500 mg of clarithromycin every 12 hours, the maximum serum concentrations and area under the curves of clarithromycin and 14-OH clarithromycin were increased compared to those achieved in healthy young adults. These changes in pharmacokinetics parallel known age-related decreases in renal function. In clinical trials, elderly patients did not have an increased incidence of adverse reactions when compared to younger patients. Consider dosage adjustment in elderly patients with severe renal impairment. Elderly patients may be more susceptible to development of torsades de pointes arrhythmias than younger patients [see Warnings and Precautions (5.3)].
Most reports of acute kidney injury with calcium channel blockers metabolized by CYP3A4 (e.g., verapamil, amlodipine, diltiazem, nifedipine) involved elderly patients 65 years of age or older [see Warnings and Precautions (5.4)].
Especially in elderly patients, there have been reports of colchicine toxicity with concomitant use of clarithromycin and colchicine, some of which occurred in patients with renal insufficiency. Deaths have been reported in some patients [see Contraindications (4.4) and Warnings and Precautions (5.4)].
Close8.6 Renal and Hepatic Impairment
Clarithromycin is principally excreted via the liver and kidney. Clarithromycin may be administered without dosage adjustment to patients with hepatic impairment and normal renal function. However, in the presence of severe renal impairment with or without coexisting hepatic impairment, decreased dosage or prolonged dosing intervals may be appropriate [see Dosage and Administration (2.6)].
-
10 OVERDOSAGEOverdosage of clarithromycin can cause gastrointestinal symptoms such as abdominal pain, vomiting, nausea, and diarrhea. Treat adverse reactions accompanying overdosage by the prompt elimination ...
Overdosage of clarithromycin can cause gastrointestinal symptoms such as abdominal pain, vomiting, nausea, and diarrhea.
Treat adverse reactions accompanying overdosage by the prompt elimination of unabsorbed drug and supportive measures. As with other macrolides, clarithromycin serum concentrations are not expected to be appreciably affected by hemodialysis or peritoneal dialysis.
Close -
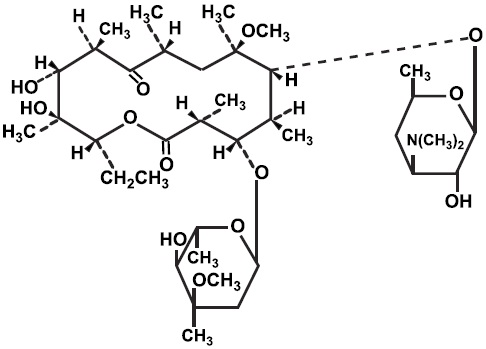
11 DESCRIPTIONClarithromycin is a semi-synthetic macrolide antimicrobial for oral use. Chemically, it is 6-0-methylerythromycin. The molecular formula is C38H69NO13, and the molecular weight is 747.96. The ...
Clarithromycin is a semi-synthetic macrolide antimicrobial for oral use. Chemically, it is 6-0-methylerythromycin. The molecular formula is C38H69NO13, and the molecular weight is 747.96. The structural formula is:
Figure 1: Structure of Clarithromycin
Clarithromycin USP is a white or almost white, crystalline powder. It is soluble in acetone, slightly soluble in methanol, ethanol, and acetonitrile, and practically insoluble in water.
Clarithromycin tablets, USP are available as immediate-release tablets.
Each film-coated tablet contains 250 mg or 500 mg of clarithromycin USP and the following inactive ingredients: microcrystalline cellulose, croscarmellose sodium, colloidal silicon dioxide, magnesium stearate, povidone, hypromellose, titanium dioxide, hydroxypropyl cellulose, iron oxide yellow, propylene glycol, vanillin, and sorbic acid.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Clarithromycin is a macrolide antimicrobial drug [see Microbiology (12.4)]. 12.3 Pharmacokinetics - Absorption - The absolute bioavailability of 250 mg clarithromycin ...
12.1 Mechanism of Action
Clarithromycin is a macrolide antimicrobial drug [see Microbiology (12.4)].
12.3 Pharmacokinetics
Absorption
The absolute bioavailability of 250 mg clarithromycin tablets was approximately 50%. For a single 500 mg dose of clarithromycin, food slightly delays the onset of clarithromycin absorption, increasing the peak time from approximately 2 to 2.5 hours. Food also increases the clarithromycin peak plasma concentration by about 24%, but does not affect the extent of clarithromycin bioavailability. Food does not affect the onset of formation of the active metabolite, 14-OH clarithromycin or its peak plasma concentration but does slightly decrease the extent of metabolite formation, indicated by an 11% decrease in area under the plasma concentration-time curve (AUC). Therefore, clarithromycin tablets may be given without regard to food. In non-fasting healthy human subjects (males and females), peak plasma concentrations were attained within 2 to 3 hours after oral dosing.
Distribution
Clarithromycin and the 14-OH clarithromycin metabolite distribute readily into body tissues and fluids. There are no data available on cerebrospinal fluid penetration. Because of high intracellular concentrations, tissue concentrations are higher than serum concentrations. Examples of tissue and serum concentrations are presented below.
Table 3. Tissue and Serum Concentrations of Clarithromycin CONCENTRATION (after 250 mg every 12 hours)
Tissue Type
Tissue
(mcg/g)
Serum
(mcg/mL)
Tonsil
1.6
0.8
Lung
8.8
1.7
Metabolism and Elimination
Steady-state peak plasma clarithromycin concentrations were attained within 3 days and were approximately 1 mcg/mL to 2 mcg/mL with a 250 mg dose administered every 12 hours and 3 mcg/mL to 4 mcg/mL with a 500 mg dose administered every 8 hours to 12 hours. The elimination half-life of clarithromycin was about 3 hours to 4 hours with 250 mg administered every 12 hours but increased to 5 hours to 7 hours with 500 mg administered every 8 hours to 12 hours. The nonlinearity of clarithromycin pharmacokinetics is slight at the recommended doses of 250 mg and 500 mg administered every 8 hours to 12 hours. With a 250 mg every 12 hours dosing, the principal metabolite, 14-OH clarithromycin, attains a peak steady-state concentration of about 0.6 mcg/mL and has an elimination half-life of 5 hours to 6 hours. With a 500 mg every 8 hours to 12 hours dosing, the peak steady-state concentration of 14-OH clarithromycin is slightly higher (up to 1 mcg/mL), and its elimination half-life is about 7 hours to 9 hours. With any of these dosing regimens, the steady-state concentration of this metabolite is generally attained within 3 days to 4 days.
After a 250 mg tablet every 12 hours, approximately 20% of the dose is excreted in the urine as clarithromycin, while after a 500 mg tablet every 12 hours, the urinary excretion of clarithromycin is somewhat greater, approximately 30%. In comparison, after an oral dose of 250 mg (125 mg/5 mL) suspension every 12 hours, approximately 40% is excreted in urine as clarithromycin. The renal clearance of clarithromycin is, however, relatively independent of the dose size and approximates the normal glomerular filtration rate. The major metabolite found in urine is 14-OH clarithromycin, which accounts for an additional 10% to 15% of the dose with either a 250 mg or a 500 mg tablet administered every 12 hours.
Specific Populations for Clarithromycin Tablets
HIV Infection
Steady-state concentrations of clarithromycin and 14-OH clarithromycin observed following administration of 500 mg doses of clarithromycin every 12 hours to adult patients with HIV infection were similar to those observed in healthy volunteers. In adult HIV-infected patients taking 500 mg or 1000 mg doses of clarithromycin every 12 hours, steady-state clarithromycin Cmax values ranged from 2 mcg/mL to 4 mcg/mL and 5 mcg/mL to 10 mcg/mL, respectively.
Hepatic Impairment
The steady-state concentrations of clarithromycin in subjects with impaired hepatic function did not differ from those in normal subjects; however, the 14-OH clarithromycin concentrations were lower in the hepatically impaired subjects. The decreased formation of 14-OH clarithromycin was at least partially offset by an increase in renal clearance of clarithromycin in the subjects with impaired hepatic function when compared to healthy subjects.
Renal Impairment
The pharmacokinetics of clarithromycin was also altered in subjects with impaired renal function [see Use in Specific Populations (8.6) and Dosage and Administration (2.6)].
Drug Interactions
Fluconazole
Following administration of fluconazole 200 mg daily and clarithromycin 500 mg twice daily to 21 healthy volunteers, the steady-state clarithromycin Cmin and AUC increased 33% and 18%, respectively. Clarithromycin exposures were increased and steady-state concentrations of 14-OH clarithromycin were not significantly affected by concomitant administration of fluconazole.
Colchicine
When a single dose of colchicine 0.6 mg was administered with clarithromycin 250 mg BID for 7 days, the colchicine Cmax increased 197% and the AUC0-∞ increased 239% compared to administration of colchicine alone.
Atazanavir
Following administration of clarithromycin (500 mg twice daily) with atazanavir (400 mg once daily), the clarithromycin AUC increased 94%, the 14-OH clarithromycin AUC decreased 70% and the atazanavir AUC increased 28%.
Ritonavir
Concomitant administration of clarithromycin and ritonavir (n = 22) resulted in a 77% increase in clarithromycin AUC and a 100% decrease in the AUC of 14-OH clarithromycin.
Saquinavir
Following administration of clarithromycin (500 mg bid) and saquinavir (soft gelatin capsules, 1200 mg tid) to 12 healthy volunteers, the steady-state saquinavir AUC and Cmax increased 177% and 187% respectively compared to administration of saquinavir alone. Clarithromycin AUC and Cmax increased 45% and 39% respectively, whereas the 14–OH clarithromycin AUC and Cmax decreased 24% and 34% respectively, compared to administration with clarithromycin alone.
Didanosine
Simultaneous administration of clarithromycin tablets and didanosine to 12 HIV-infected adult patients resulted in no statistically significant change in didanosine pharmacokinetics.
Zidovudine
Following administration of clarithromycin 500 mg tablets twice daily with zidovudine 100 mg every 4 hours, the steady-state zidovudine AUC decreased 12% compared to administration of zidovudine alone (n=4). Individual values ranged from a decrease of 34% to an increase of 14%. When clarithromycin tablets were administered two to four hours prior to zidovudine, the steady-state zidovudine Cmax increased 100% whereas the AUC was unaffected (n=24).
Omeprazole
Clarithromycin 500 mg every 8 hours was given in combination with omeprazole 40 mg daily to healthy adult subjects. The steady-state plasma concentrations of omeprazole were increased (Cmax, AUC0-24, and t½ increases of 30%, 89%, and 34%, respectively), by the concomitant administration of clarithromycin.
The plasma levels of clarithromycin and 14–OH clarithromycin were increased by the concomitant administration of omeprazole. For clarithromycin, the mean Cmax was 10% greater, the mean Cmin was 27% greater, and the mean AUC0-8 was 15% greater when clarithromycin was administered with omeprazole than when clarithromycin was administered alone. Similar results were seen for 14–OH clarithromycin, the mean Cmax was 45% greater, the mean Cmin was 57% greater, and the mean AUC0-8 was 45% greater. Clarithromycin concentrations in the gastric tissue and mucus were also increased by concomitant administration of omeprazole.
Clarithromycin Tissue Concentrations 2 hours after Dose (mcg/mL)/(mcg/g)
Treatment
N
antrum
fundus
N
Mucus
Clarithromycin
5
10.48 ± 2.01
20.81 ± 7.64
4
4.15 ± 7.74
Clarithromycin + Omeprazole
5
19.96 ± 4.71
24.25 ± 6.37
4
39.29 ± 32.79
Theophylline
In two studies in which theophylline was administered with clarithromycin (a theophylline sustained-release formulation was dosed at either 6.5 mg/kg or 12 mg/kg together with 250 or 500 mg q12h clarithromycin), the steady-state levels of Cmax, Cmin, and the area under the serum concentration time curve (AUC) of theophylline increased about 20%.
Midazolam
When a single dose of midazolam was co-administered with clarithromycin tablets (500 mg twice daily for 7 days), midazolam AUC increased 174% after intravenous administration of midazolam and 600% after oral administration.
For information about other drugs indicated in combination with clarithromycin, refer to their full prescribing information, CLINICAL PHARMACOLOGY section.
Close12.4 Microbiology
Mechanism of Action
Clarithromycin exerts its antibacterial action by binding to the 50S ribosomal subunit of susceptible bacteria resulting in inhibition of protein synthesis.
Resistance
The major routes of resistance are modification of the 23S rRNA in the 50S ribosomal subunit to insensitivity or drug efflux pumps. Beta-lactamase production should have no effect on clarithromycin activity.
Most isolates of methicillin-resistant and oxacillin-resistant staphylococci are resistant to clarithromycin.
If H. pylori is not eradicated after treatment with clarithromycin-containing combination regimens, patients may develop clarithromycin resistance in H. pylori isolates. Therefore, for patients who fail therapy, clarithromycin susceptibility testing should be done, if possible. Patients with clarithromycin-resistant H. pylori should not be treated with any of the following: omeprazole/clarithromycin dual therapy; omeprazole/clarithromycin/amoxicillin triple therapy; lansoprazole/clarithromycin/amoxicillin triple therapy; or other regimens which include clarithromycin as the sole antibacterial agent.
Antimicrobial Activity
Clarithromycin has been shown to be active against most of the isolates of the following microorganisms both in vitro and in clinical infections [see Indications and Usage (1)].
Gram-Positive Bacteria
- •
- Staphylococcus aureus
- •
- Streptococcus pneumoniae
- •
- Streptococcus pyogenes
Gram-Negative Bacteria
- •
- Haemophilus influenzae
- •
- Haemophilus parainfluenzae
- •
- Moraxella catarrhalis
Other Microorganisms
- •
- Chlamydophila pneumoniae
- •
- Helicobacter pylori
- •
- Mycobacterium avium complex (MAC) consisting of M. avium and M. intracellulare
- •
- Mycoplasma pneumoniae
At least 90 percent of the microorganisms listed below exhibit in vitro minimum inhibitory concentrations (MICs) less than or equal to the clarithromycin susceptible MIC breakpoint for organisms of similar type to those shown in Table 11. However, the efficacy of clarithromycin in treating clinical infections due to these microorganisms has not been established in adequate and well-controlled clinical trials.
Gram-Positive Bacteria
- •
- Streptococcus agalactiae
- •
- Streptococci (Groups C, F, G)
- •
- Viridans group streptococci
Gram-Negative Bacteria
- •
- Legionella pneumophila
- •
- Pasteurella multocida
Anaerobic Bacteria
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria, and associated test methods and quality control standards recognized by FDA for this drug, please see: http://www.fda.gov/STIC.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Mutagenesis - The following in vitro mutagenicity tests have been conducted with clarithromycin: • Salmonella/Mammalian ...
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Mutagenesis
The following in vitro mutagenicity tests have been conducted with clarithromycin:- •
- Salmonella/Mammalian Microsomes Test
- •
- Bacterial Induced Mutation Frequency Test
- •
- In Vitro Chromosome Aberration Test
- •
- Rat Hepatocyte DNA Synthesis Assay
- •
- Mouse Lymphoma Assay
- •
- Mouse Dominant Lethal Study
- •
- Mouse Micronucleus Test
All tests had negative results except the in vitro chromosome aberration test which was positive in one test and negative in another. In addition, a bacterial reverse-mutation test (Ames test) has been performed on clarithromycin metabolites with negative results.
Impairment of Fertility
Fertility and reproduction studies have shown that daily doses of up to 160 mg/kg/day to male and female rats caused no adverse effects on the estrous cycle, fertility, parturition, or number and viability of offspring. Plasma levels in rats after 150 mg/kg/day were twice the human serum levels.
Testicular atrophy occurred in rats at doses 7 times, in dogs at doses 3 times, and in monkeys at doses 8 times greater than the maximum human daily dose (on a body surface area basis).
Close13.2 Animal Toxicology and/or Pharmacology
Corneal opacity occurred in dogs at doses 12 times and in monkeys at doses 8 times greater than the maximum human daily dose (on a body surface area basis). Lymphoid depletion occurred in dogs at doses 3 times greater than and in monkeys at doses 2 times greater than the maximum human daily dose (on a body surface area basis).
-
14 CLINICAL STUDIES14.1 Mycobacterial Infections - Prophylaxis of Mycobacterial Infections - A randomized, double-blind clinical trial (trial 3) compared clarithromycin 500 mg twice a day to placebo in patients ...
14.1 Mycobacterial Infections
Prophylaxis of Mycobacterial Infections
A randomized, double-blind clinical trial (trial 3) compared clarithromycin 500 mg twice a day to placebo in patients with CDC-defined AIDS and CD4 counts less than 100 cells/μL. This trial accrued 682 patients from November 1992 to January 1994, with a median CD4 cell count at entry of 30 cells/mcL. Median duration of clarithromycin was 10.6 months vs. 8.2 months for placebo. More patients in the placebo arm than the clarithromycin arm discontinued prematurely from the trial (75.6% and 67.4%, respectively). However, if premature discontinuations due to Mycobacterium avium complex (MAC) or death are excluded, approximately equal percentages of patients on each arm (54.8%) on clarithromycin and 52.5% on placebo) discontinued study drug early for other reasons. The trial was designed to evaluate the following endpoints:
1. MAC bacteremia, defined as at least one positive culture for Mycobacterium avium complex bacteria from blood or another normally sterile site
2. Survival
3. Clinically significant disseminated MAC disease, defined as MAC bacteremia accompanied by signs or symptoms of serious MAC infection, including fever, night sweats, weight loss, anemia, or elevations in liver function tests
MAC Bacteremia
In patients randomized to clarithromycin, the risk of MAC bacteremia was reduced by 69% compared to placebo. The difference between groups was statistically significant (p < 0.001). On an intent-to-treat basis, the one-year cumulative incidence of MAC bacteremia was 5.0% for patients randomized to clarithromycin and 19.4% for patients randomized to placebo. While only 19 of the 341 patients randomized to clarithromycin developed MAC, 11 of these cases were resistant to clarithromycin. The patients with resistant MAC bacteremia had a median baseline CD4 count of 10 cells/mm3 (range 2 cells/mm3 to 25 cells/mm3). Information regarding the clinical course and response to treatment of the patients with resistant MAC bacteremia is limited. The 8 patients who received clarithromycin and developed susceptible MAC bacteremia had a median baseline CD4 count of 25 cells/mm3 (range 10 cells/mm3 to 80 cells/mm3). Comparatively, 53 of the 341 placebo patients developed MAC; none of these isolates were resistant to clarithromycin. The median baseline CD4 count was 15 cells/mm3 (range 2 cells/mm3 to 130 cells/mm3) for placebo patients that developed MAC.
Survival
A statistically significant survival benefit of clarithromycin compared to placebo was observed (see Figure 3 and Table 13). Since the analysis at 18 months includes patients no longer receiving prophylaxis the survival benefit of clarithromycin may be underestimated.
Figure 3. Survival of All Randomized AIDS Patients Over Time in Trial 3
Table 4. Mortality Rates at 18 months in Trial 3 Mortality Rates
Reduction in Mortality Rates on Clarithromycin
Placebo
Clarithromycin
6 month
9.4%
6.5%
31%
12 month
29.7%
20.5%
31%
18 month
46.4%
37.5%
20%
Clinically Significant Disseminated MAC Disease
In association with the decreased incidence of MAC bacteremia, patients in the group randomized to clarithromycin showed reductions in the signs and symptoms of disseminated MAC disease, including fever, night sweats, weight loss, and anemia.
Treatment of Mycobacterial Infections
Dose-Ranging Monotherapy Trials in Adult AIDS Patients with MAC
Two randomized clinical trials (Trials 1 and 2) compared different dosages of clarithromycin in patients with CDC-defined AIDS and CD4 counts less than100 cells/mcL. These trials accrued patients from May 1991 to March 1992. Trial 500 was a randomized, double-blind trial; trial 577 was an open-label compassionate use trial. Both trials used 500 mg and 1000 mg twice daily dosing of clarithromycin; trial 1 also had a 2000 mg twice daily clarithromycin group. Trial 1 enrolled 154 adult patients and trial 2 enrolled 469 adult patients. The majority of patients had CD4 cell counts less than 50 cells/mcL at study entry. The trials were designed to evaluate the following end points:
1. Change in MAC bacteremia or blood cultures negative for M. avium.
2. Change in clinical signs and symptoms of MAC infection including one or more of the following: fever, night sweats, weight loss, diarrhea, splenomegaly, and hepatomegaly.
The results for trial 1 are described below. The trial 2 results were similar to the results of trial 1.
MAC Bacteremia
Decreases in MAC bacteremia or negative blood cultures were seen in the majority of patients in all clarithromycin dosage groups. The mean reductions in MAC colony forming units (CFU) from baseline after 4 weeks of therapy in the 1000 mg (n=32) twice daily and 2000 mg (n=26) twice daily regimen was 2.3 Log CFU compared to 1.5 Log CFU in the clarithromycin 500 mg twice daily (n=35) regimen. A separate trial with a four-drug regimen2 (ciprofloxacin, ethambutol, rifampicin, and clofazimine) had a mean reduction of 1.4 Log CFU.
Clinical outcomes evaluated with the different dosing regimens of clarithromycin monotherapy are shown in Table 14. The 1000 mg and 2000 mg twice daily doses showed significantly better control of bacteremia during the first four weeks of therapy. No significant differences were seen beyond that point. All of the isolates had MIC less than 8 mcg/mL at pre-treatment. Relapse was almost always accompanied by an increase in MIC.
Table 5. Outcome with the Different Dosing Regimens of Clarithromycin Outcome
Clarithromycin 500 mg twice daily
Clarithromycin 1000 mg twice daily
Clarithromycin 2000 mg twice daily
One or more negative blood cultures at any time during acute therapy
61% (30/49)
59% (29/49)
52% (25/48)
Two or more negative blood cultures during acute therapy sustained through study day 84
25% (12/49)
25% (12/49)
8% (4/48)
Death or discontinuation by day 84
23% (11/49)
37% (18/49)
56% (27/48)
Relapse by day 84
14% (7/49)
12% (6/49)
13% (6/48)
Median time to first negative culture (in days)
54
41
29
Median time to first decrease of at least 1 log CFU (in days)
29
16
15
Median time to first positive culture or study discontinuation following the first negative culture (in days)
43
59
43
Clinically Significant Disseminated MAC Disease
Among patients experiencing night sweats prior to therapy, 84% showed resolution or improvement at some point during the 12 weeks of clarithromycin at 500 mg to 2000 mg twice daily doses. Similarly, 77% of patients reported resolution or improvement in fevers at some point. Response rates for clinical signs of MAC are given in Table 15 below.
The median duration of response, defined as improvement or resolution of clinical signs and symptoms, was 2 weeks to 6 weeks.
Since the trial was not designed to determine the benefit of monotherapy beyond 12 weeks, the duration of response may be underestimated for the 25% to 33% of patients who continued to show clinical response after 12 weeks.
Table 6. Response Rates for Clinical Signs of MAC During 6 Weeks to 12 Weeks of Treatment Resolution of Fever
Resolution of Night Sweats
Clarithromycin twice daily dose (mg)
% ever afebrile
% afebrile
6 weeks or more
Clarithromycin twice daily dose (mg)
% ever resolving
% resolving
6 weeks or more
500
67%
23%
500
85%
42%
1000
67%
12%
1000
70%
33%
2000
62%
22%
2000
72%
36%
Weight Gain Greater Than 3%
Hemoglobin Increase Greater Than 1 gm
Clarithromycin twice daily dose (mg)
% ever gaining
% gaining
6 weeks or more
Clarithromycin twice daily dose (mg)
% ever increasing
% increasing
6 weeks or more
500
33%
14%
500
58%
26%
1000
26%
17%
1000
37%
6%
2000
26%
12%
2000
62%
18%
Survival
Median survival time from trial entry (trial 1) was 249 days at the 500 mg twice daily dose compared to 215 days with the 1000 mg twice daily dose. However, during the first 12 weeks of therapy, there were 2 deaths in 53 patients in the 500 mg twice daily group versus 13 deaths in 51 patients in the 1000 mg twice daily group. The reason for this apparent mortality difference is not known. Survival in the two groups was similar beyond 12 weeks. The median survival times for these dosages were similar to recent historical controls with MAC when treated with combination therapies.2
Median survival time from entry in trial 2 was 199 days for the 500 mg twice a day dose and 179 days for the 1000 mg twice a day dose. During the first four weeks of therapy, while patients were maintained on their originally assigned dose, there were 11 deaths in 255 patients taking 500 mg twice daily and 18 deaths in 214 patients taking 1000 mg twice daily.
Dosage-Ranging Monotherapy Trials in Pediatric AIDS Patients with MAC
Trial 4 was a pediatric trial of 3.75 mg/kg, 7.5 mg/kg, and 15 mg/kg of clarithromycin twice daily in patients with CDC-defined AIDS and CD4‑ counts less than 100 cells/mcL. The trial enrolled 25 patients between the ages of 1 to 20. The trial evaluated the same endpoints as in the adult trials 1 and 2. Results with the 7.5 mg/kg twice daily dose in the pediatric trial were comparable to those for the 500 mg twice daily regimen in the adult trials.
Combination Therapy in AIDS Patients with Disseminated MAC
Trial 5 compared the safety and efficacy of clarithromycin in combination with ethambutol versus clarithromycin in combination with ethambutol and clofazimine for the treatment of disseminated MAC (dMAC) infection. This 24-week trial enrolled 106 patients with AIDS and dMAC, with 55 patients randomized to receive clarithromycin and ethambutol, and 51 patients randomized to receive clarithromycin, ethambutol, and clofazime. Baseline characteristics between treatment arms were similar with the exception of median CFU counts being at least 1 log higher in the clarithromycin, ethambutol, and clofazime arm.
Compared to prior experience with clarithromycin monotherapy, the two-drug regimen of clarithromycin and ethambutol extended the time to microbiologic relapse, largely through suppressing the emergence of clarithromycin resistant strains. However, the addition of clofazimine to the regimen added no additional microbiologic or clinical benefit. Tolerability of both multidrug regimens was comparable with the most common adverse events being gastrointestinal in nature. Patients receiving the clofazimine-containing regimen had reduced survival rates; however, their baseline mycobacterial colony counts were higher. The results of this trial support the addition of ethambutol to clarithromycin for the treatment of initial dMAC infections but do not support adding clofazimine as a third agent.
14.2 Otitis Media
Otitis Media Trial of Clarithromycin vs. Oral Cephalosporin
In a controlled clinical trial of pediatric patients with acute otitis media performed in the United States, where significant rates of beta-lactamase producing organisms were found, clarithromycin was compared to an oral cephalosporin. In this trial, strict evaluability criteria were used to determine clinical response. For the 223 patients who were evaluated for clinical efficacy, the clinical success rate (i.e., cure plus improvement) at the post-therapy visit was 88% for clarithromycin and 91% for the cephalosporin.
In a smaller number of patients, microbiologic determinations were made at the pre-treatment visit. The presumptive bacterial eradication/clinical cure outcomes (i.e., clinical success) are shown in Table 16.
Table 7. Clinical Success Rates of Otitis Media Treatment by Pathogen Pathogen
Clinical Success Rates
Clarithromycin
Oral Cephalosporin
S. pneumoniae
13/15 (87%)
4/5
H. influenzaea
10/14 (71%)
3/4
M. catarrhalis
4/5
1/1
S. pyogenes
3/3
0/1
All Pathogens Combined
30/37 (81%)
8/11 (73%)
a None of the H. influenzae isolated pre-treatment was resistant to clarithromycin; 6% were resistant to the control agent.
Otitis Media Trials of Clarithromycin vs. Antimicrobial/Beta-lactamase Inhibitor
In two other controlled clinical trials of acute otitis media performed in the United States, where significant rates of beta-lactamase producing organisms were found, clarithromycin was compared to an oral antimicrobial agent that contained a specific beta-lactamase inhibitor. In these trials, strict evaluability criteria were used to determine the clinical responses. In the 233 patients who were evaluated for clinical efficacy, the combined clinical success rate (i.e., cure and improvement) at the post-therapy visit was 91% for both clarithromycin and the control.
For the patients who had microbiologic determinations at the pre-treatment visit, the presumptive bacterial eradication/clinical cure outcomes (i.e., clinical success) are shown in Table 17.
Table 8. Clinical Success Rates of Acute Otitis Media Treatment by Pathogen Clinical Success Rates
PATHOGEN
Clarithromycin
Antimicrobial/Beta-lactamase Inhibitor
S. pneumoniae
43/51 (84%)
55/56 (98%)
H. influenzaea
36/45 (80%)
31/33 (94%)
M. catarrhalis
9/10 (90%)
6/6
S. pyogenes
3/3
5/5
All Pathogens Combined
91/109 (83%)
97/100 (97%)
a Of the H. influenzae isolated pre-treatment, 3% were resistant to clarithromycin and 10% were resistant to the control agent.
Close14.3 H. pylori Eradication to Decrease the Risk of Duodenal Ulcer Recurrence
Clarithromycin + Lansoprazole and Amoxicillin
Two U.S. randomized, double-blind clinical trials (trial 6 and trial 7) in patients with H. pylori and duodenal ulcer disease (defined as an active ulcer or history of an active ulcer within one year) evaluated the efficacy of clarithromycin 500 mg twice daily in combination with lansoprazole 30 mg twice daily and amoxicillin 1 gm twice daily as 14-day triple therapy for eradication of H. pylori.
H. pylori eradication was defined as two negative tests (culture and histology) at 4 weeks to 6 weeks following the end of treatment.
The combination of clarithromycin plus lansoprazole and amoxicillin as triple therapy was effective in eradication of H. pylori (see results in Table 18). Eradication of H. pylori has been shown to reduce the risk of duodenal ulcer recurrence.
A randomized, double-blind clinical trial (trial 8) performed in the U.S. in patients with H. pylori and duodenal ulcer disease (defined as an active ulcer or history of an ulcer within one year) compared the efficacy of clarithromycin in combination with lansoprazole and amoxicillin as triple therapy for 10 days and 14 days. This trial established that the 10-day triple therapy was equivalent to the 14-day triple therapy in eradicating H. pylori (see results in Table 18).
Table 18. H. pylori Eradication Rates-Triple Therapy (clarithromycin/lansoprazole/amoxicillin) Percent of Patients Cured [95% Confidence Interval] (number of patients)
Trial
Duration
Triple Therapy
Evaluable Analysisa
Triple Therapy
Intent-to-Treat Analysisb
Trial 6
14 days
92c [80 to 97.7]
(n = 48)
86c [73.3 to 93.5]
(n = 55)
Trial 7
14 days
86d [75.7 to 93.6]
(n = 66)
83d [72 to 90.8]
(n = 70)
Trial 8e
14 days
85 [77 to 91]
(N = 113)
82 [73.9 to 88.1]
(N = 126)
10 days
84 [76 to 89.8]
(N = 123)
81 [73.9 to 87.6]
(N = 135)
a Based on evaluable patients with confirmed duodenal ulcer (active or within one year) and H. pylori infection at baseline defined as at least two of three positive endoscopic tests from CLOtest (Delta West LTD., Bentley, Australia), histology, and/or culture. Patients were included in the analysis if they completed the trial. Additionally, if patients were dropped out of the trial due to an adverse reaction related to the drug, they were included in the analysis as evaluable failures of therapy.
b Patients were included in the analysis if they had documented H. pylori infection at baseline as defined above and had a confirmed duodenal ulcer (active or within one year). All dropouts were included as failures of therapy.
c (p < 0.05) versus clarithromycin/lansoprazole and lansoprazole/amoxicillin dual therapy.
d (p < 0.05) versus clarithromycin/amoxicillin dual therapy.
e The 95% confidence interval for the difference in eradication rates, 10-day minus 14-day, is (-10.5, 8.1) in the evaluable analysis and (-9.7, 9.1) in the intent-to-treat analysis.
Clarithromycin + Omeprazole and Amoxicillin Therapy
Three U.S., randomized, double-blind clinical trials in patients with H. pylori infection and duodenal ulcer disease (n = 558) compared clarithromycin plus omeprazole and amoxicillin to clarithromycin plus amoxicillin. Two trials (trials 9 and 10) were conducted in patients with an active duodenal ulcer, and the third trial (trial 11) was conducted in patients with a duodenal ulcer in the past 5 years, but without an ulcer present at the time of enrollment. The dosage regimen in the trials was clarithromycin 500 mg twice a day plus omeprazole 20 mg twice a day plus amoxicillin 1 gram twice a day for 10 days. In trials 9 and 10, patients who took the omeprazole regimen also received an additional 18 days of omeprazole 20 mg once a day. Endpoints studied were eradication of H. pylori and duodenal ulcer healing (trials 9 and 10 only). H. pylori status was determined by CLOtest®, histology, and culture in all three trials. For a given patient, H. pylori was considered eradicated if at least two of these tests were negative, and none was positive. The combination of clarithromycin plus omeprazole and amoxicillin was effective in eradicating H. pylori (see results in Table 19).
Table 19. H. pylori Eradication Rates: % of Patients Cured [95% Confidence Interval]
Clarithromycin + omeprazole + amoxicillin
Clarithromycin + amoxicillin
Per-Protocola
Intent-to-Treatb
Per-Protocola
Intent-to-Treatb
Trial 9
c77 [64, 86]
(n = 64)
69 [57, 79]
(n = 80)
43 [31, 56]
(n = 67)
37 [27, 48]
(n = 84)
Trial 10
c78 [67, 88]
(n = 65)
73 [61, 82]
(n = 77)
41 [29, 54]
(n = 68)
36 [26, 47]
(n = 84)
Trial 11
c90 [80, 96]
(n = 69)
83 [74, 91]
(n = 84)
33 [24, 44]
(n = 93)
32 [23, 42]
(n = 99)
a Patients were included in the analysis if they had confirmed duodenal ulcer disease (active ulcer trials 9 and 10; history of ulcer within 5 years, trial 11) and H. pylori infection at baseline defined as at least two of three positive endoscopic tests from CLOtest®, histology, and/or culture. Patients were included in the analysis if they completed the trial. Additionally, if patients dropped out of the trial due to an adverse reaction related to the study drug, they were included in the analysis as failures of therapy. The impact of eradication on ulcer recurrence has not been assessed in patients with a past history of ulcer.
b Patients were included in the analysis if they had documented H. pylori infection at baseline and had confirmed duodenal ulcer disease. All dropouts were included as failures of therapy.
c p < 0.05 versus clarithromycin plus amoxicillin.
Clarithromycin + Omeprazole Therapy
Four randomized, double-blind, multi-center trials (trials 12, 13, 14, and 15) evaluated clarithromycin 500 mg three times a day plus omeprazole 40 mg once a day for 14 days, followed by omeprazole 20 mg once a day (trials 12, 13, and 15) or by omeprazole 40 mg once a day (trial 14) for an additional 14 days in patients with active duodenal ulcer associated with H. pylori. Trials 12 and 13 were conducted in the U.S. and Canada and enrolled 242 and 256 patients, respectively. H. pylori infection and duodenal ulcer were confirmed in 219 patients in trial 12 and 228 patients in trial 13. These trials compared the combination regimen to omeprazole and clarithromycin monotherapies. Trials 14 and 15 were conducted in Europe and enrolled 154 and 215 patients, respectively. H. pylori infection and duodenal ulcer were confirmed in 148 patients in trial 14 and 208 patients in trial 15. These trials compared the combination regimen to omeprazole monotherapy. The results for the efficacy analyses for these trials are described in Tables 20, 21, and 22.
Duodenal Ulcer Healing
The combination of clarithromycin and omeprazole was as effective as omeprazole alone for healing duodenal ulcer (see Table 20).
Table 20. End-of-Treatment Ulcer Healing Rates Percent of Patients Healed (n/N)
Trial
Clarithromycin + Omeprazole
Omeprazole
Clarithromycin
U.S. Trials
Trial 13
94% (58/62)a
88% (60/68)
71% (49/69)
Trial 12
88% (56/64)a
85% (55/65)
64% (44/69)
Non-U.S. Trials
Trial 15
99% (84/85)
95% (82/86)
N/A
Trial 14b
100% (64/64)
99% (71/72)
N/A
a p < 0.05 for clarithromycin + omeprazole versus clarithromycin monotherapy.
b In trial 14 patients received omeprazole 40 mg daily for days 15 to 28.
Eradication of H. pylori Associated with Duodenal Ulcer
The combination of clarithromycin and omeprazole was effective in eradicating H. pylori (see Table 21). H. pylori eradication was defined as no positive test (culture or histology) at 4 weeks following the end of treatment, and two negative tests were required to be considered eradicated. In the per-protocol analysis, the following patients were excluded: dropouts, patients with major protocol violations, patients with missing H. pylori tests post-treatment, and patients that were not assessed for H. pylori eradication at 4 weeks after the end of treatment because they were found to have an unhealed ulcer at the end of treatment.
Table 21. H. pylori Eradication Rates (Per-Protocol Analysis) at 4 to 6 weeks Percent of Patients Cured (n/N)
Trial
Clarithromycin + Omeprazole
Omeprazole
Clarithromycin
U.S. Trials
Trial 13
64% (39/61)a,b
0% (0/59)
39% (17/44)
Trial 12
74% (39/53)a,b
0% (0/54)
31% (13/42)
Non-U.S. Trials
Trial 15
74% (64/86)b
1% (1/90)
N/A
Trial 14
83% (50/60)b
1% (1/74)
N/A
a Statistically significantly higher than clarithromycin monotherapy (p < 0.05).
b Statistically significantly higher than omeprazole monotherapy (p < 0.05).
Duodenal Ulcer Recurrence
Ulcer recurrence at 6-months and at 12 months following the end of treatment was assessed for patients in whom ulcers were healed post-treatment (see the results in Table 22). Thus, in patients with duodenal ulcer associated with H. pylori infection, eradication of H. pylori reduced ulcer recurrence.
Table 22. Duodenal Ulcer Recurrence at 6 months and 12 months in Patients with Healed Ulcers
H. pylori Negative at 4 to 6 Weeks
H. pylori Positive at 4 to 6 Weeks
U.S. Trials Recurrence at 6 Months
Trial 100
Clarithromycin + Omeprazole
6% (2/34)
56% (9/16)
Omeprazole
(0/0)
71% (35/49)
Clarithromycin
12% (2/17)
32% (7/22)
Trial 067
Clarithromycin + Omeprazole
38% (11/29)
50% (6/12)
Omeprazole
(0/0)
67% (31/46)
Clarithromycin
18% (2/11)
52% (14/27)
Non-U.S. Trials Recurrence at 6 Months
Trial 058
Clarithromycin + Omeprazole
6% (3/53)
24% (4/17)
Omeprazole
0% (0/3)
55% (39/71)
Trial 812b
Clarithromycin + Omeprazole
5% (2/42)
0% (0/7)
Omeprazole
0% (0/1)
54% (32/59)
Non-U.S. Trials Recurrence at 12 Months in Trial 14
Clarithromycin + Omeprazole
3% (1/40)
0% (0/6)
Omeprazole
0% (0/1)
67% (29/43)
-
15 REFERENCES1. Winkel P, Hilden J, Hansen JF, Kastrup J, Kolmos HJ, Kjøller E, et al. Clarithromycin for stable coronary heart disease increases all-cause and cardiovascular mortality and cerebrovascular ...
- 1.
- Winkel P, Hilden J, Hansen JF, Kastrup J, Kolmos HJ, Kjøller E, et al. Clarithromycin for stable coronary heart disease increases all-cause and cardiovascular mortality and cerebrovascular morbidity over 10 years in the CLARICOR randomised, blinded clinical trial. Int J Cardiol 2015;182:459-65.
- 2.
- Kemper CA, et al. Treatment of Mycobacterium avium Complex Bacteremia in AIDS with a Four-Drug Oral Regimen. Ann Intern Med. 1992;116:466-472.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGClarithromycin Tablets USP, 500 mg are light yellow colored, oval shaped, biconvex film-coated tablets, with ‘D’ debossed on one side and ‘63’ on the other side. Bottles of ...
Clarithromycin Tablets USP, 500 mg are light yellow colored, oval shaped, biconvex film-coated tablets, with ‘D’ debossed on one side and ‘63’ on the other side.
Bottles of 28 NDC 82804-141-28
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Close -
17 PATIENT COUNSELING INFORMATIONProvide the following instructions or information about clarithromycin to patients: Important Administration Instructions - Advise patients that clarithromycin tablets can be taken with or ...
Provide the following instructions or information about clarithromycin to patients:
Important Administration Instructions
Advise patients that clarithromycin tablets can be taken with or without food and can be taken with milk.
Drug Interactions
Advise patients that clarithromycin may interact with some drugs; therefore, advise patients to report to their healthcare provider the use of any other medications.
Diarrhea
Advise patients that diarrhea is a common problem caused by antibacterials including clarithromycin which usually ends when the antibacterial is discontinued. Sometimes after starting treatment with antibacterials, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibacterial. If this occurs, instruct patients to contact their healthcare provider as soon as possible.
Embryo-Fetal Toxicity
Advise females of reproductive potential that that if pregnancy occurs while taking this drug, there is a potential hazard to the fetus [see Warnings and Precautions (5.7) and Use in Specific Populations (8.1)].
Antibacterial Resistance
Counsel patients that antibacterial drugs including clarithromycin should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When clarithromycin is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by clarithromycin or other antibacterial drugs in the future.
Potential for Dizziness, Vertigo and Confusion
There are no data on the effect of clarithromycin on the ability to drive or use machines. However, counsel patients regarding the potential for dizziness, vertigo, confusion and disorientation, which may occur with the clarithromycin. The potential for these adverse reactions should be taken into account before patients drive or use machines.
Risk of Mortality in Patients with Coronary Disease Years After Clarithromycin Treatment
Advise patients who have coronary artery disease to continue medications and lifestyle modifications for their coronary artery disease because clarithromycin may be associated with increased risk for mortality years after the end of clarithromycin treatment.
The brands listed are trademarks of their respective owners and are not trademarks of Aurobindo Pharma Limited. The makers of these brands are not affiliated with and do not endorse Aurobindo Pharma Limited or its products.Distributed by:
Aurobindo Pharma USA, Inc.
279 Princeton-Hightstown Road
East Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma Limited
Hyderabad-500 032, IndiaRepackaged by:
Close
Proficient Rx LP
Thousand Oaks, CA 91320
Revised: 10/2023 -
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 500 mg (28 Tablets Bottle)NDC 82804-141-28 - Rx only - Clarithromycin - Tablets, USP - 500 mg - 28 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information
CLARITHROMYCIN clarithromycin tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:82804-141(NDC:65862-226) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLARITHROMYCIN (UNII: H1250JIK0A) (CLARITHROMYCIN - UNII:H1250JIK0A) CLARITHROMYCIN 500 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE K30 (UNII: U725QWY32X) HYPROMELLOSE 2910 (15 MPA.S) (UNII: 36SFW2JZ0W) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) VANILLIN (UNII: CHI530446X) SORBIC ACID (UNII: X045WJ989B) Product Characteristics Color YELLOW (Light Yellow) Score no score Shape OVAL (Biconvex) Size 18mm Flavor Imprint Code D;63 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82804-141-28 28 in 1 BOTTLE; Type 0: Not a Combination Product 09/17/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065489 07/25/2012 Labeler - Proficient Rx LP (079196022)
CloseEstablishment Name Address ID/FEI Business Operations Proficient Rx LP 079196022 REPACK(82804-141) , RELABEL(82804-141)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
CLARITHROMYCIN tablet, film coated
Number of versions: 1
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Sep 27, 2024 | 1 (current) | download |
Get Label RSS Feed for this Drug
CLARITHROMYCIN tablet, film coated
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=88c7ded8-333c-408d-b693-a72881710f59
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
CLARITHROMYCIN tablet, film coated
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 82804-141-28 |