Label: KENGREAL- cangrelor injection, powder, lyophilized, for solution
- NDC Code(s): 10122-620-01, 10122-620-10
- Packager: Chiesi USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 21, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use KENGREAL safely and effectively. See full prescribing information for KENGREAL. KENGREAL® (cangrelor) for injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
KENGREAL is indicated as an adjunct to percutaneous coronary intervention (PCI) to reduce the risk of periprocedural myocardial infarction (MI), repeat coronary revascularization, and stent ...
-
2 DOSAGE AND ADMINISTRATION
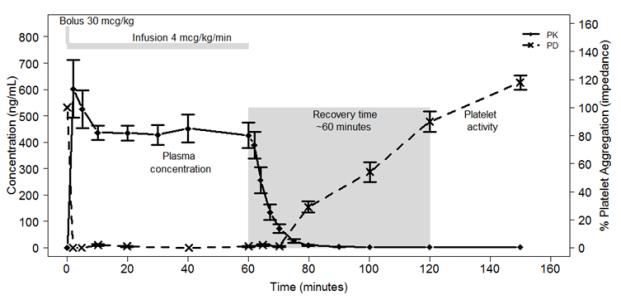
2.1 - Recommended Dosing - The recommended dosage of KENGREAL is a 30 mcg/kg IV bolus followed immediately by a 4 mcg/kg/min IV infusion. Initiate the bolus infusion prior to PCI. The ...
-
3 DOSAGE FORMS AND STRENGTHS
For Injection: 50 mg of KENGREAL lyophilized powder in a single-use 10 mL glass vial for reconstitution.
-
4 CONTRAINDICATIONS
4.1 - Significant Active Bleeding - KENGREAL is contraindicated in patients with significant active bleeding [see Warnings and Precautions (5.1) and Adverse Reactions ...
-
5 WARNINGS AND PRECAUTIONS
5.1 - Bleeding - Drugs that inhibit platelet P2Y12 function, including KENGREAL, increase the risk of bleeding. In CHAMPION PHOENIX bleeding events of all severities were more common ...
-
6 ADVERSE REACTIONS
The following adverse reactions are also discussed elsewhere in the labeling: Bleeding [see Warnings and Precautions (5.1)] 6.1 Clinical Trials Experience - Because clinical trials are ...
-
7 DRUG INTERACTIONS
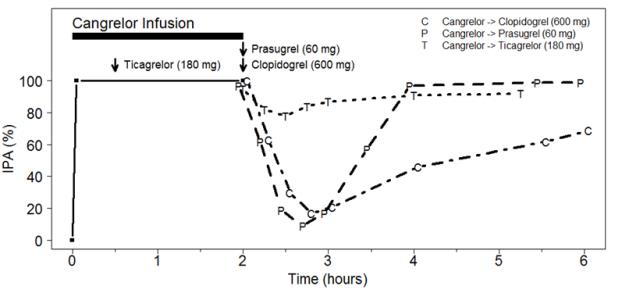
7.1 Thienopyridines - Clopidogrel or prasugrel administered during KENGREAL infusion will have no antiplatelet effect until the next dose is administered. Therefore, administer ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 - Pregnancy - Risk Summary - There are no available data on cangrelor use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse ...
-
10 OVERDOSAGE
There is no specific treatment to reverse the antiplatelet effect of KENGREAL but the effect is gone within one hour after the drug is discontinued. In clinical trials, 36 patients received an ...
-
11 DESCRIPTION
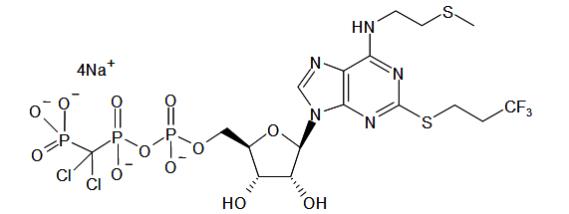
KENGREAL is a direct-acting P2Y12 platelet receptor inhibitor that blocks adenosine diphosphate (ADP)-induced platelet activation and aggregation. The chemical structure is similar to adenosine ...
-
12 CLINICAL PHARMACOLOGY
12.1 - Mechanism of Action - Cangrelor is a direct P2Y12 platelet receptor inhibitor that blocks ADP-induced platelet activation and aggregation. Cangrelor binds selectively and ...
-
13NONCLINICAL TOXICOLOGY
13.1 - Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - No carcinogenicity studies were conducted. Mutagenesis - Cangrelor was non-mutagenic and non-clastogenic in ...
-
14 CLINICAL STUDIES
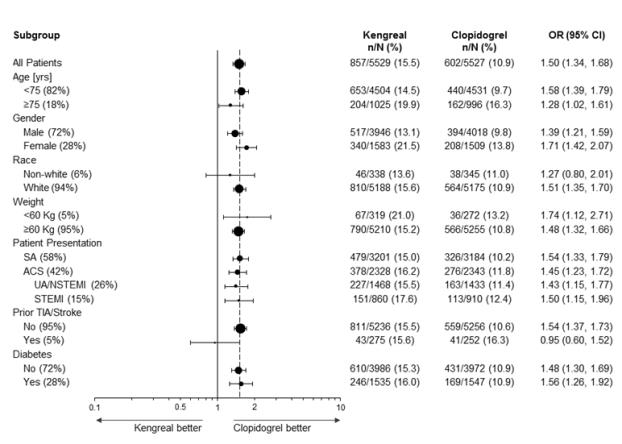
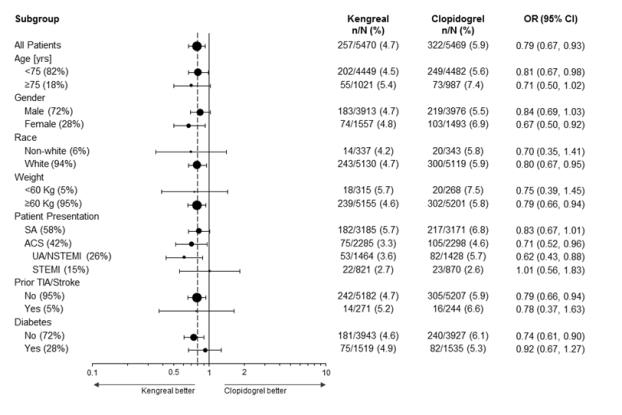
14.1 - CHAMPION PHOENIX Trial - The CHAMPION PHOENIX trial was intended to test whether faster platelet inhibition with cangrelor at the time of PCI would reduce the rate of ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
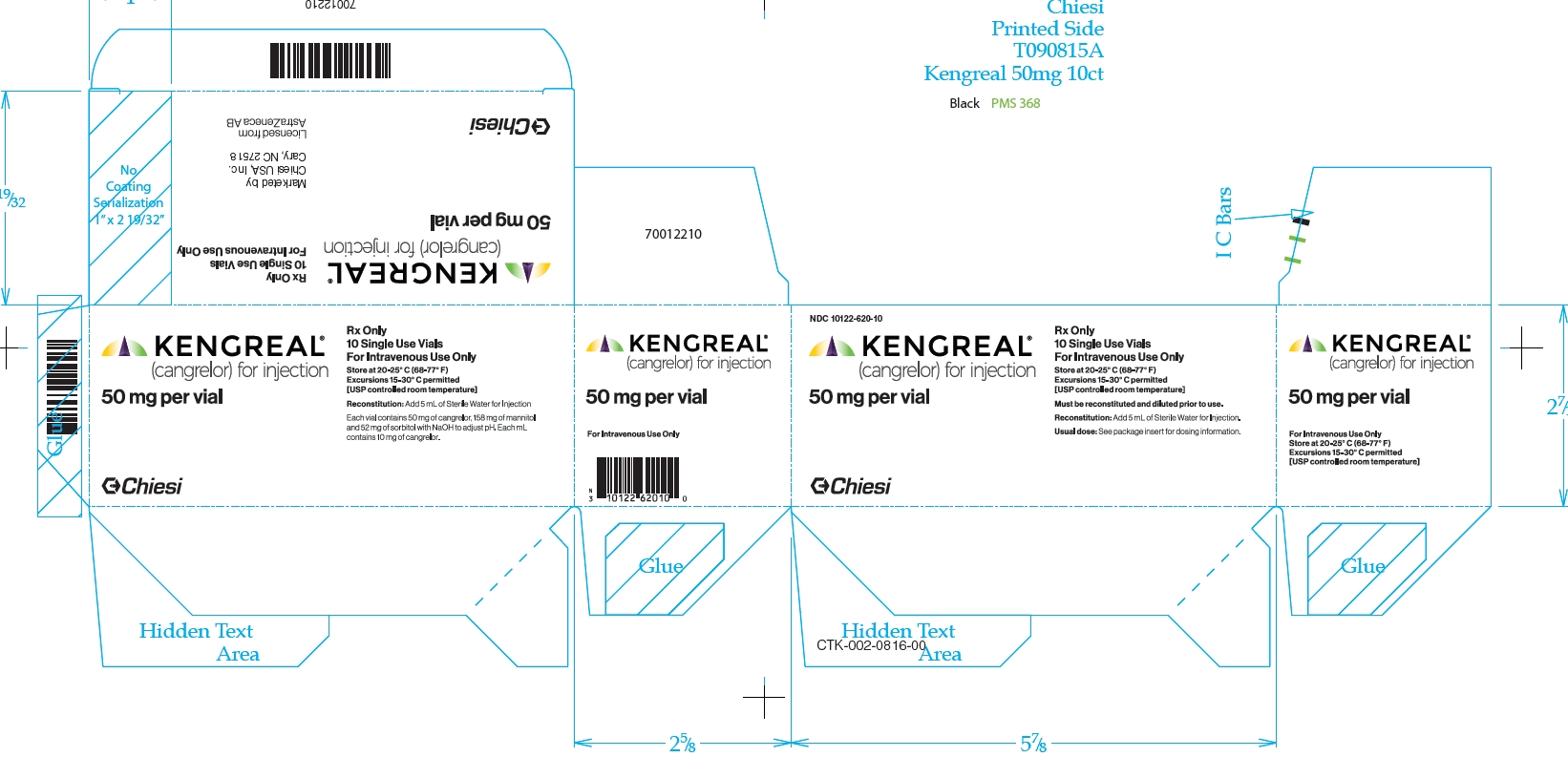
KENGREAL is supplied as a sterile lyophilized powder in single-use 10 mL vials. NDC # 10122-620-01: 10 mL vial containing 50 mg cangrelor - NDC # 10122-620-10: 10 count of 10 mL vials containing ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - NDC 10122-620-10 - Kengreal® (cangrelor) for injection - 50 mg per vial - Rx Only - 10 Single Use Vials
-
INGREDIENTS AND APPEARANCEProduct Information