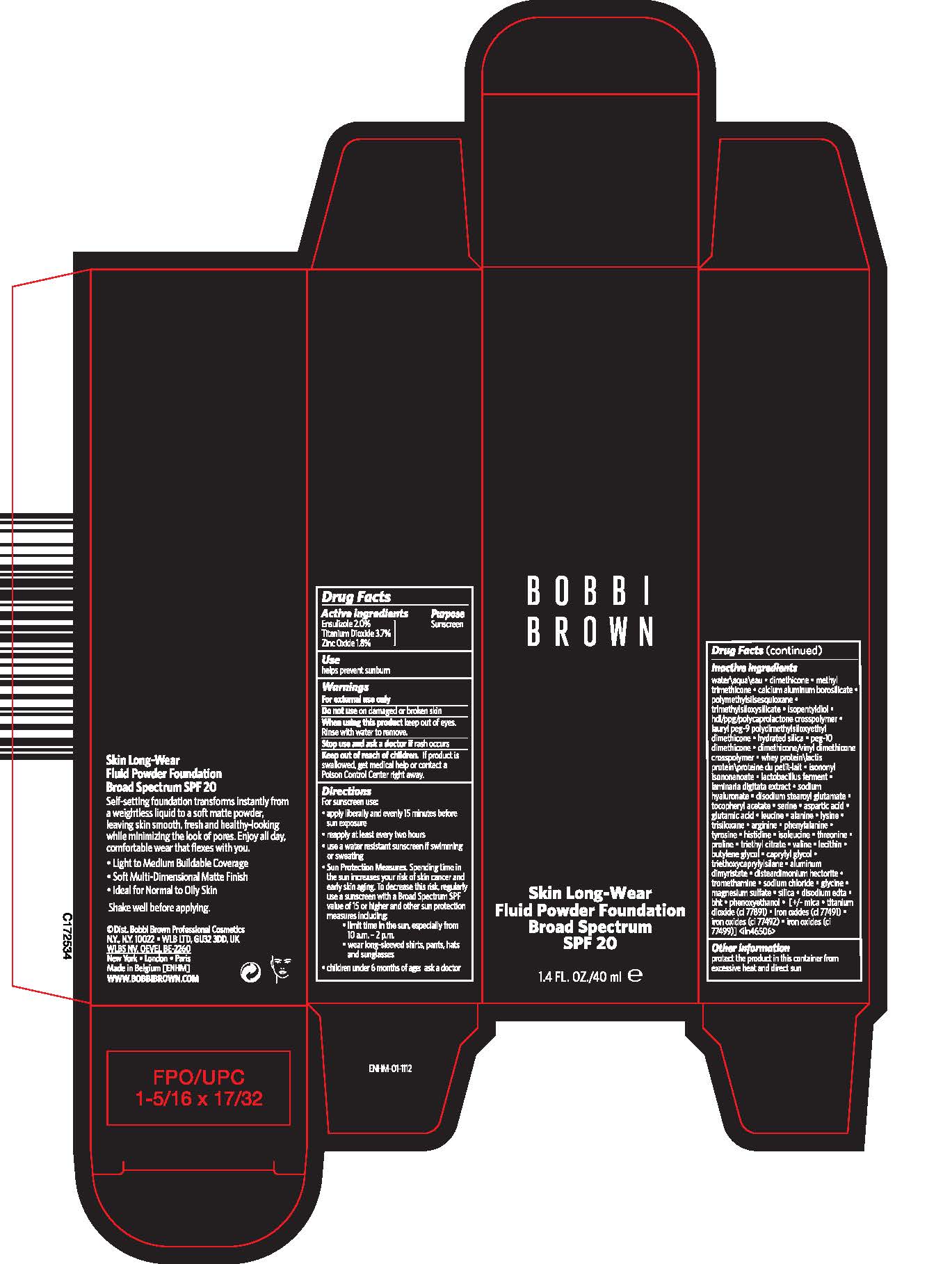
Label: SKIN LONG-WEAR FLUID POWDER FOUNDATION BROAD SPECTRUM SPF 20- ensulizole, titanium dioxide, and zinc oxide liquid
- NDC Code(s): 64141-030-01
- Packager: Bobbi Brown Professional Cosmetics Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Use
- Warnings
-
Directions
For sunscreen use:
- apply liberally and evenly 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
-
Inactive ingredients
water\aqua\eau ▪ dimethicone ▪ methyl trimethicone ▪ calcium aluminum borosilicate ▪ polymethylsilsesquioxane ▪ trimethylsiloxysilicate ▪ isopentyldiol ▪ hdi/ppg/polycaprolactone crosspolymer ▪ lauryl peg-9 polydimethylsiloxyethyl dimethicone ▪ hydrated silica ▪ peg-10 dimethicone ▪ dimethicone/vinyl dimethicone crosspolymer ▪ whey protein\lactis protein\proteine du petit-lait ▪ isononyl isononanoate ▪ lactobacillus ferment ▪ laminaria digitata extract ▪ sodium hyaluronate ▪ disodium stearoyl glutamate ▪ tocopheryl acetate ▪ serine ▪ aspartic acid ▪ glutamic acid ▪ leucine ▪ alanine ▪ lysine ▪ trisiloxane ▪ arginine ▪ phenylalanine ▪ tyrosine ▪ histidine ▪ isoleucine ▪ threonine ▪ proline ▪ triethyl citrate ▪ valine ▪ lecithin ▪ butylene glycol ▪ caprylyl glycol ▪ triethoxycaprylylsilane ▪ aluminum dimyristate ▪ disteardimonium hectorite ▪ tromethamine ▪ sodium chloride ▪ glycine ▪ magnesium sulfate ▪ silica ▪ disodium edta ▪ bht ▪ phenoxyethanol ▪ [+/- mica ▪ titanium dioxide (ci 77891) ▪ iron oxides (ci 77491) ▪ iron oxides (ci 77492) ▪ iron oxides (ci 77499)] <iln46506>
- Other information
- PRINCIPAL DISPLAY PANEL - 40 ml Tube Carton
-
INGREDIENTS AND APPEARANCE
SKIN LONG-WEAR FLUID POWDER FOUNDATION BROAD SPECTRUM SPF 20
ensulizole, titanium dioxide, and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64141-030 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ENSULIZOLE (UNII: 9YQ9DI1W42) (ENSULIZOLE - UNII:9YQ9DI1W42) ENSULIZOLE 20 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 37 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 18 mg in 1 mL Inactive Ingredients Ingredient Name Strength METHYL TRIMETHICONE (UNII: S73ZQI0GXM) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ISOPENTYLDIOL (UNII: 19NOL5474Q) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) HYDRATED SILICA (UNII: Y6O7T4G8P9) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) WHEY (UNII: 8617Z5FMF6) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) LIMOSILACTOBACILLUS REUTERI (UNII: 9913I24QEE) LAMINARIA DIGITATA (UNII: 15E7C67EE8) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SERINE (UNII: 452VLY9402) ASPARTIC ACID (UNII: 30KYC7MIAI) GLUTAMIC ACID (UNII: 3KX376GY7L) LEUCINE (UNII: GMW67QNF9C) ALANINE (UNII: OF5P57N2ZX) LYSINE (UNII: K3Z4F929H6) TRISILOXANE (UNII: 9G1ZW13R0G) ARGININE (UNII: 94ZLA3W45F) PHENYLALANINE (UNII: 47E5O17Y3R) TYROSINE (UNII: 42HK56048U) HISTIDINE (UNII: 4QD397987E) ISOLEUCINE (UNII: 04Y7590D77) THREONINE (UNII: 2ZD004190S) PROLINE (UNII: 9DLQ4CIU6V) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) VALINE (UNII: HG18B9YRS7) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CAPRYLYL GLYCOL (UNII: 00YIU5438U) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TROMETHAMINE (UNII: 023C2WHX2V) SODIUM CHLORIDE (UNII: 451W47IQ8X) GLYCINE (UNII: TE7660XO1C) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64141-030-01 1 in 1 CARTON 10/16/2019 12/31/2024 1 40 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/16/2019 12/31/2024 Labeler - Bobbi Brown Professional Cosmetics Inc. (627131279) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations Estee Lauder N.V. 370151326 manufacture(64141-030) , pack(64141-030) , label(64141-030)