Label: PREMARIN- conjugated estrogens injection, powder, lyophilized, for solution
- NDC Code(s): 0046-0749-05
- Packager: Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 30, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONSpecially prepared for Intravenous & Intramuscular use - Rx only
-
BOXED WARNING
(What is this?)Estrogen-Alone Therapy - Endometrial Cancer - There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens. Adding a progestin to estrogen therapy has ...
WARNING: ENDOMETRIAL CANCER, CARDIOVASCULAR DISORDERS, BREAST CANCER and PROBABLE DEMENTIA
Estrogen-Alone Therapy
Endometrial Cancer
There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens. Adding a progestin to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer. Adequate diagnostic measures, including directed or random endometrial sampling when indicated, should be undertaken to rule out malignancy in postmenopausal women with undiagnosed persistent or recurring abnormal genital bleeding. (See WARNINGS, Malignant Neoplasms, Endometrial cancer.)
Cardiovascular Disorders and Probable Dementia
Estrogen-alone therapy should not be used for the prevention of cardiovascular disease or dementia. (See CLINICAL STUDIES and WARNINGS, Cardiovascular Disorders and Probable Dementia.)
The Women's Health Initiative (WHI) estrogen-alone substudy reported increased risks of stroke and deep vein thrombosis (DVT) in postmenopausal women (50 to 79 years of age) during 7.1 years of treatment with daily oral conjugated estrogens (CE) [0.625 mg]-alone, relative to placebo. (See CLINICAL STUDIES and WARNINGS, Cardiovascular disorders.)
The WHI Memory Study (WHIMS) estrogen-alone ancillary study of the WHI reported an increased risk of developing probable dementia in postmenopausal women 65 years of age or older during 5.2 years of treatment with daily CE (0.625 mg)-alone, relative to placebo. It is unknown whether this finding applies to younger postmenopausal women. (See CLINICAL STUDIES and WARNINGS, Probable Dementia and PRECAUTIONS, Geriatric Use.)
In the absence of comparable data, these risks should be assumed to be similar for other doses of CE and other dosage forms of estrogens.
Estrogens with or without progestins should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
CloseEstrogen Plus Progestin Therapy
Cardiovascular Disorders and Probable Dementia
Estrogen plus progestin therapy should not be used for the prevention of cardiovascular disease or dementia. (See CLINICAL STUDIES and WARNINGS, Cardiovascular disorders and Probable Dementia.)
The WHI estrogen plus progestin substudy reported increased risks of DVT, pulmonary embolism (PE), stroke and myocardial infarction (MI) in postmenopausal women (50 to 79 years of age) during 5.6 years of treatment with daily oral CE (0.625 mg) combined with medroxyprogesterone acetate (MPA) [2.5 mg], relative to placebo. (See CLINICAL STUDIES and WARNINGS, Cardiovascular disorders.)
The WHIMS estrogen plus progestin ancillary study of the WHI reported an increased risk of developing probable dementia in postmenopausal women 65 years of age or older during 4 years of treatment with daily CE (0.625 mg) combined with MPA (2.5 mg), relative to placebo. It is unknown whether this finding applies to younger postmenopausal women. (See CLINICAL STUDIES and WARNINGS, Probable Dementia and PRECAUTIONS, Geriatric Use.)
Breast Cancer
The WHI estrogen plus progestin substudy also demonstrated an increased risk of invasive breast cancer. (See CLINICAL STUDIES and WARNINGS, Malignant Neoplasms, Breast cancer.)
In the absence of comparable data, these risks should be assumed to be similar for other doses of CE and MPA, and other combinations and dosage forms of estrogens and progestins.
Estrogens with or without progestins should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
-
DESCRIPTIONPremarin Intravenous (conjugated estrogens, USP) for injection contains a mixture of conjugated estrogens obtained exclusively from natural sources, occurring as the sodium salts of water-soluble ...
-
CLINICAL PHARMACOLOGY Endogenous estrogens are largely responsible for the development and maintenance of the female reproductive system and secondary sexual characteristics. Although circulating estrogens exist in a ...
-
CLINICAL STUDIESWomen's Health Initiative Studies - The Women's Health Initiative (WHI) enrolled approximately 27,000 predominantly healthy postmenopausal women in two substudies to assess the risks and benefits ...
-
INDICATIONS AND USAGEPremarin Intravenous (conjugated estrogens, USP) for injection is indicated in the treatment of abnormal uterine bleeding due to hormonal imbalance in the absence of organic pathology. Premarin ...
-
CONTRAINDICATIONSPremarin Intravenous therapy should not be used in individuals with any of the following conditions: 1. Undiagnosed abnormal genital bleeding. 2. Known, suspected, or history of breast ...
-
WARNINGSSee BOXED WARNINGS. Premarin Intravenous for injection is indicated for short-term use. However, warnings, precautions and adverse reactions associated with oral Premarin treatment should be taken ...
-
PRECAUTIONSA. General - Premarin Intravenous for injection is indicated for short-term use. However, warnings, precautions and adverse reactions associated with oral Premarin treatment should be taken into ...
-
ADVERSE REACTIONSSee BOXED WARNINGS, WARNINGS, and PRECAUTIONS. Premarin Intravenous for injection is indicated for short-term use. However, the warnings, precautions and adverse reactions associated with oral ...
-
OVERDOSAGEOverdosage of estrogen may cause nausea, vomiting, breast tenderness, abdominal pain, drowsiness and fatigue, and withdrawal bleeding may occur in women. Treatment of overdose consists of ...
-
DOSAGE AND ADMINISTRATIONFor treatment of abnormal uterine bleeding due to hormonal imbalance in the absence of organic pathology: One 25 mg injection, intravenously or intramuscularly. Intravenous use is preferred since ...
-
DIRECTIONS FOR STORAGE AND RECONSTITUTIONSTORAGE BEFORE RECONSTITUTION - Store package in refrigerator, 2° to 8°C (36° to 46°F). TO RECONSTITUTE - Reconstitute Premarin Intravenous with 5 mL of Sterile Water for Injection, USP ...
-
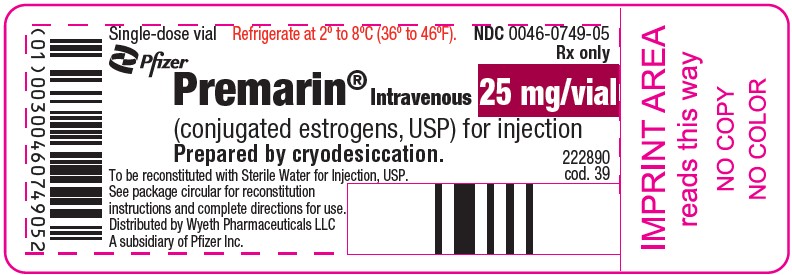
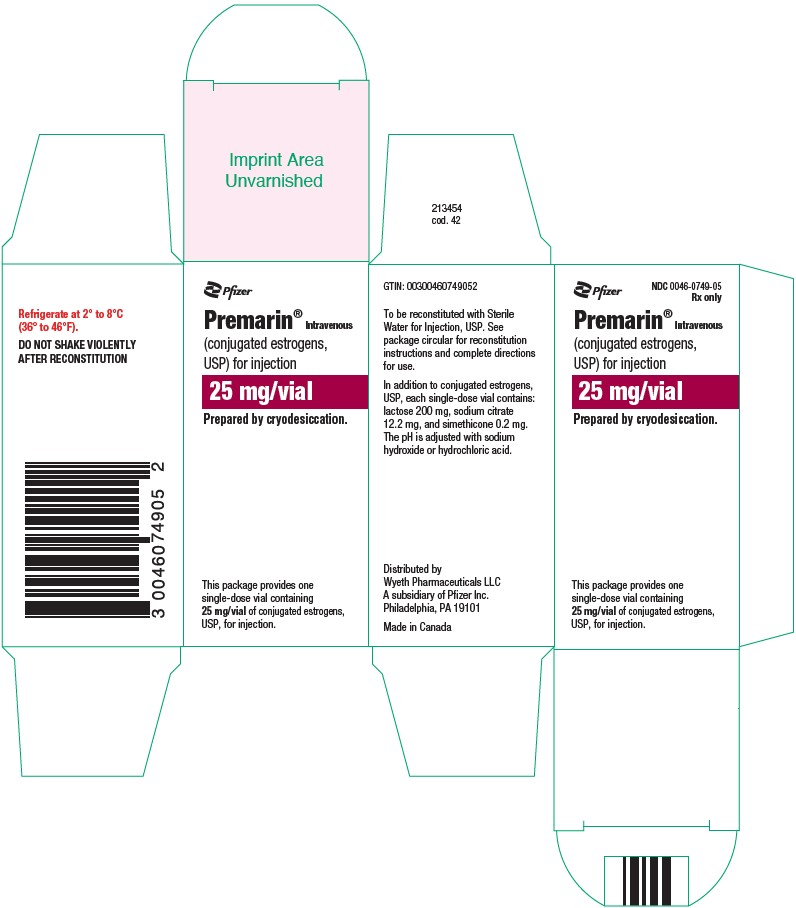
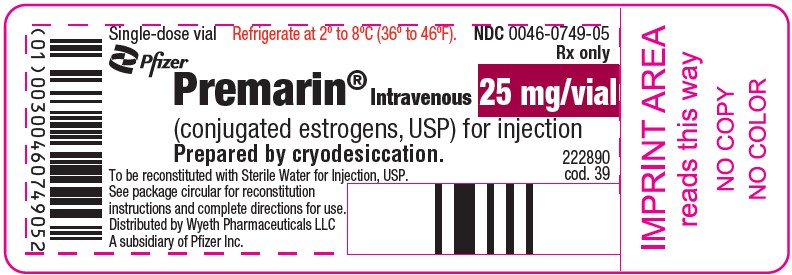
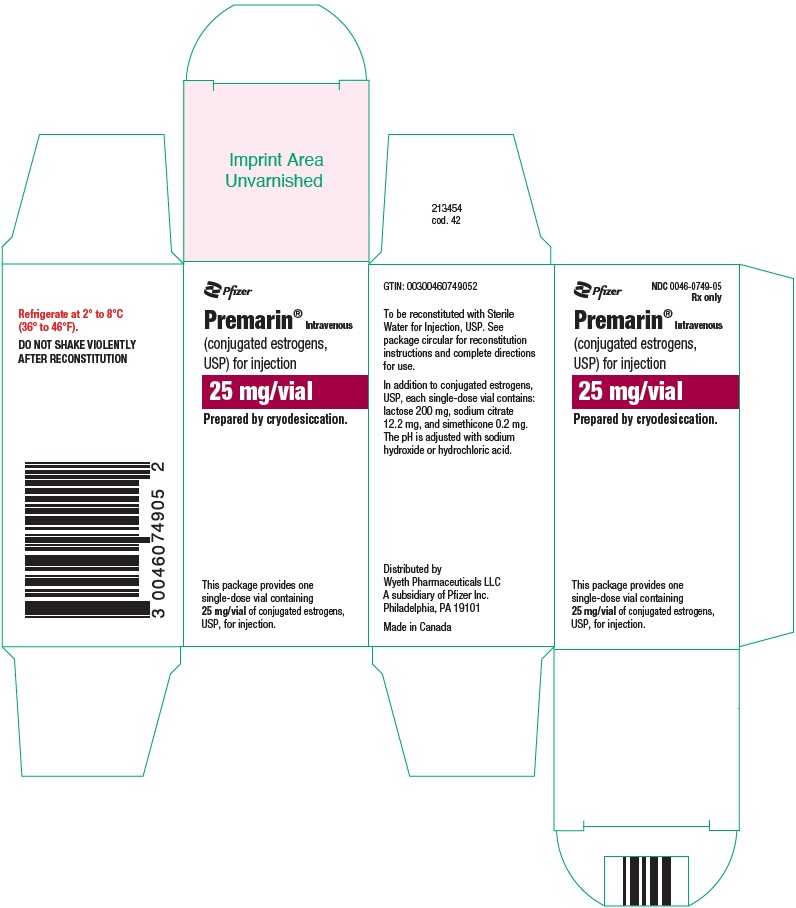
HOW SUPPLIEDNDC 0046-0749-05–Each package provides one single-dose vial containing 25 mg/vial of conjugated estrogens, USP, for injection (also lactose 200 mg, sodium citrate 12.2 mg, and simethicone 0.2 mg) ...
-
PATIENT INFORMATIONPremarin Intravenous (conjugated estrogens, USP) for injection - Read this PATIENT INFORMATION which describes the benefit and major risks of your treatment, as well as how and when treatment ...
-
PATIENT INFORMATIONPremarin - Intravenous - (conjugated estrogens, USP) for injection - Rx only - Read this PATIENT INFORMATION which describes the benefit and major risks of your treatment, as well as how and ...
-
PRINCIPAL DISPLAY PANEL - 25 mg Vial LabelSingle-dose vial - Refrigerate at 2° to 8°C (36° to 46°F). NDC 0046-0749-05 - Rx only - Pfizer - Premarin® Intravenous - (conjugated estrogens, USP) for injection - Prepared by cryodesiccation. 25 mg/vial - To ...
-
PRINCIPAL DISPLAY PANEL - 25 mg Vial CartonPfizer - NDC 0046-0749-05 - Rx only - Premarin® Intravenous - (conjugated estrogens, USP) for injection - 25 mg/vial - Prepared by cryodesiccation. This package provides one - single-dose vial containing - 25 ...
-
INGREDIENTS AND APPEARANCEProduct Information