Label: CVS CORTISONE- hydrocortisone cream
- NDC Code(s): 59779-099-02, 59779-099-03, 59779-099-04
- Packager: CVS Pharmacy
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
-
Uses
- temporarily relieves itching associated with minor skin irritations, inflammation, and rashes due to:
- eczema
- psoriasis
- poison ivy, oak, sumac
- insect bites
- detergents
- jewelry
- cosmetics
- soaps
- seborrheic dermatitis
- temporarily relieves external anal and genital itching
- other uses of this product should only be under the advice and supervision of a doctor
- temporarily relieves itching associated with minor skin irritations, inflammation, and rashes due to:
-
Warnings
For external use only
Do not use
- in the genital area if you have a vaginal discharge. Consult a doctor.
- for the treatment of diaper rash. Consult a doctor.
When using this product
- avoid contact with eyes
- do not use more than directed unless told to do so by a doctor
- do not put directly into the rectum by using fingers or any mechanical device or applicator
-
Directions
-
for itching of skin irritation, inflammation, and rashes:
- adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
-
for external anal and genital itching, adults:
- when practical, clean the affected area with mild soap and warm water and rinse thoroughly
- gently dry by patting or blotting with toilet tissue or a soft cloth before applying
- apply to affected area not more than 3 to 4 times daily
- children under 12 years of age: ask a doctor
-
for itching of skin irritation, inflammation, and rashes:
- Other information
-
Inactive Ingredients
aloe barbadensis, cetearyl alcohol/sodium lauryl sulfate/sodium cetearyl sulfate, chamomile (anthemis nobilis) oil, citric acid, corn (zea mays) oil, glycerin, glyceryl stearate, isopropyl palmitate, maltodextrin, methylparaben, mineral oil, paraffin, petrolatum, propylene glycol, propylparaben, purified water, stearyl alcohol, vitamin A (retinyl palmitate), vitamin D (cholecalciferol), vitamin E (tocopheryl acetate).
- Questions?
- SPL UNCLASSIFIED SECTION
-
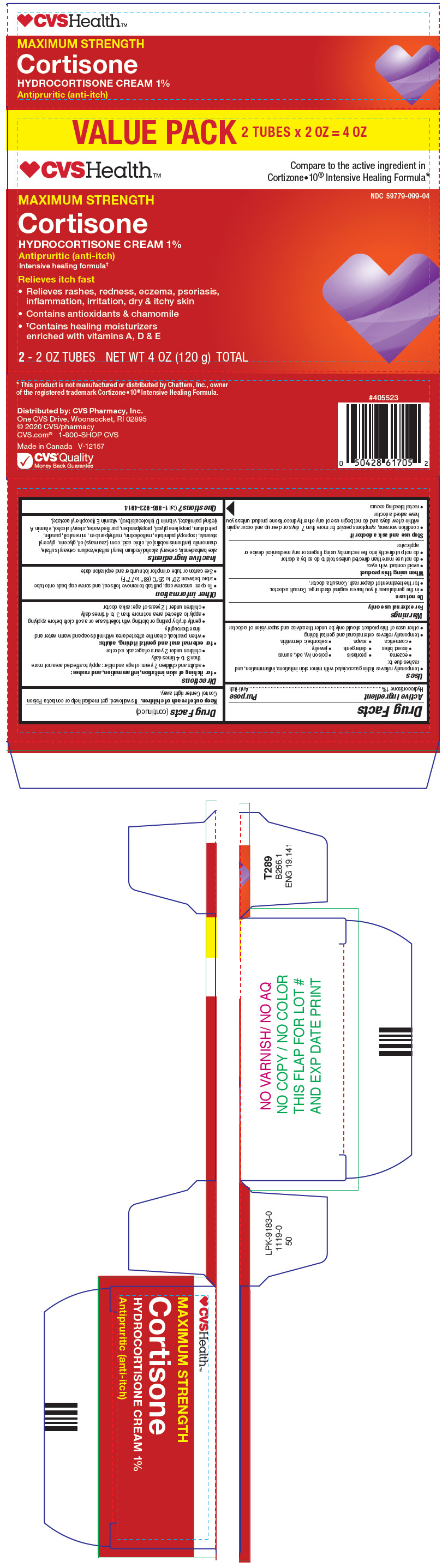
PRINCIPAL DISPLAY PANEL - 2 60 g Tube Carton
VALUE PACK 2 TUBES x 2 OZ = 4 OZ
CVSHealth ™
Compare to the active ingredient in
Cortizone•10 ®Intensive Healing Formula*NDC 59779-099-04
MAXIMUM STRENGTH
Cortisone
HYDROCORTISONE CREAM 1%Antipruritic (anti-itch)
Intensive healing formula †Relieves itch fast
- Relieves rashes, redness, eczema, psoriasis,
inflammation, irritation, dry & itchy skin - Contains antioxidants & chamomile
-
†Contains healing moisturizers
enriched with vitamins A, D & E
2 - 2 OZ TUBES NET WT 4 OZ (120 g) TOTAL
- Relieves rashes, redness, eczema, psoriasis,
-
INGREDIENTS AND APPEARANCE
CVS CORTISONE
hydrocortisone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59779-099 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 1 g in 100 g Inactive Ingredients Ingredient Name Strength CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM CETOSTEARYL SULFATE (UNII: 7ZBS06BH4B) CHAMOMILE FLOWER OIL (UNII: 60F80Z61A9) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) CORN OIL (UNII: 8470G57WFM) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) MALTODEXTRIN (UNII: 7CVR7L4A2D) METHYLPARABEN (UNII: A2I8C7HI9T) MINERAL OIL (UNII: T5L8T28FGP) PARAFFIN (UNII: I9O0E3H2ZE) PETROLATUM (UNII: 4T6H12BN9U) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) CHOLECALCIFEROL (UNII: 1C6V77QF41) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59779-099-03 1 in 1 CARTON 12/28/2012 1 60 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:59779-099-02 1 in 1 CARTON 12/28/2012 2 30 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:59779-099-04 2 in 1 CARTON 11/15/2019 3 60 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 08/23/1995 Labeler - CVS Pharmacy (062312574) Establishment Name Address ID/FEI Business Operations Taro Pharmaceuticals Inc. 206263295 manufacture(59779-099)