Label: RIMANTALIST- rimantadine hydrochloride, arginine kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 52959-305-30, 68405-110-26 - Packager: Physician Therapeutics LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated August 1, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
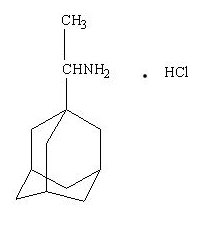
DESCRIPTIONDESCRIPTION - Rimantadine hydrochloride is a synthetic antiviral drug available as a 100 mg film-coated tablet. Each film-coated tablet contains 100 mg of rimantadine hydrochloride. In addition, each ...
-
CLINICAL PHARMACOLOGYCLINICAL PHARMACOLOGY - MECHANISM OF ACTION - The mechanism of action of rimantadine is not fully understood. Rimantadine appears to exert its inhibitory effect early in the viral replicative ...
-
MICROBIOLOGYMICROBIOLOGY - Rimantadine inhibits the replication in cell culture of influenza A virus isolates from each of the three antigenic subtypes, i.e., H1N1, H2N2 and H3N2, that have been isolated from ...
-
PHARMACOKINETICSPHARMACOKINETICS - Although the pharmacokinetic profile of rimantadine hydrochloride has been described, no pharmacodynamic data establishing a correlation between plasma concentration and its ...
-
INDICATIONS & USAGEINDICATIONS AND USAGE - Rimantadine hydrochloride tablet is indicated for the prophylaxis and treatment of illness caused by various strains of influenza A virus in adults (17 years and older) ...
-
CONTRAINDICATIONSCONTRAINDICATIONS - Rimantadine hydrochloride is contraindicated in patients with known hypersensitivity to drugs of the adamantane class, including rimantadine and amantadine.
-
PRECAUTIONSPRECAUTIONS - GENERAL - An increased incidence of seizures has been reported in patients with a history of epilepsy who received the related drug amantadine. In clinical trials of rimantadine ...
-
DRUG INTERACTIONSDRUG INTERACTIONS - Acetaminophen - Rimantadine hydrochloride, 100 mg, was given twice daily for 13 days to 12 healthy volunteers. On day 11, acetaminophen (650 mg four times daily) was started and ...
-
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITYCARCINOGENESIS, MUTAGENESIS, AND IMPAIRMENT OF FERTILITY - Carcinogenesis - Oral administration of rimantadine to rats for 2 years at doses up to 100 mg/kg/d [approximately 11-14 times the maximum ...
-
PREGNANCYPREGNANCY - Teratogenic Effects - Pregnancy Category C - There are no adequate and well-controlled studies in pregnant women. Rimantadine is reported to cross the placenta in mice. Rimantadine has ...
-
NURSING MOTHERSNURSING MOTHERS - Rimantadine hydrochloride should not be administered to nursing mothers because of the adverse effects noted in offspring of rats treated with rimantadine during the nursing ...
-
PEDIATRIC USEPEDIATRIC USE - In children (1 year to 16 years of age), rimantadine hydrochloride is recommended for the prophylaxis of influenza A. The safety and effectiveness of rimantadine hydrochloride in ...
-
ADVERSE REACTIONSADVERSE REACTIONS - In 1,027 patients treated with rimantadine hydrochloride in controlled clinical trials at the recommended dose of 200 mg daily, the most frequently reported adverse events ...
-
GERIATRIC USEGERIATRIC USE - Approximately 200 patients over the age of 64 were evaluated for safety in controlled clinical trials with rimantadine hydrochloride. Geriatric subjects who received either 200 mg ...
-
OVERDOSAGEOVERDOSAGE - As with any overdose, supportive therapy should be administered as indicated. Overdoses of a related drug, amantadine, have been reported with adverse reactions consisting of ...
-
DOSAGE & ADMINISTRATIONDOSAGE AND ADMINISTRATION - FOR PROPHYLAXIS IN ADULTS AND CHILDREN - Adults (17 years and older) The recommended adult dose of rimantadine hydrochloride is 100 mg twice a day. Study durations ...
-
HOW SUPPLIEDHOW SUPPLIED - Rimantadine Hydrochloride Tablets, 100 mg—Each orange, oval, film-coated, convex-faced tablet is debossed with a "G" on one side and "1911" on the other side. Bottles of ...
-
STORAGE AND HANDLINGStore at 20°C to 25°C (68° to 77°F) [see USP Controlled Room Temperature]
-
REFERENCESREFERENCES - 1. Belshe RB, Burk B, Newman F, et al. J. Infect. Dis. 1989; 159(3):430-435. 2. Sim IS, Cerruti RL, Connell EV. J. Resp. Dis. 1989(Suppl): S46-S51. 3. Hayden FG, Belshe RB, Clover ...
-
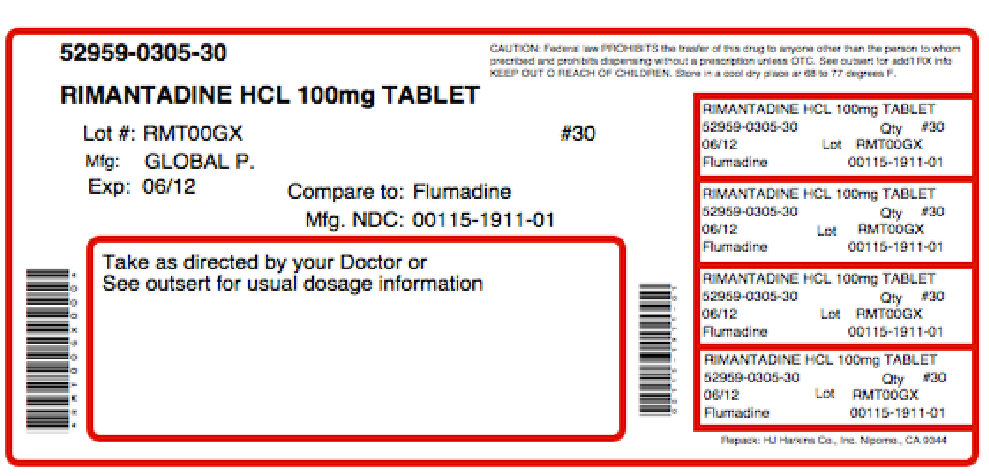
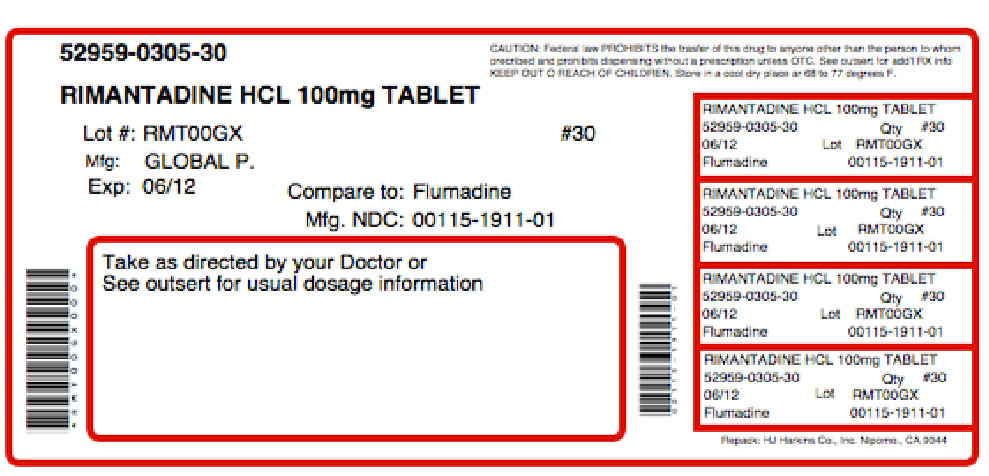
PRINCIPAL DISPLAY PANELGLOBAL NDC 0115-1911-01 Rimantadine Hydrochloride Tablets 100 mg Rx only 100 Tablets USUAL DOSAGE: See accompanying outsert for complete prescribing information. Each tablet contains 100 ...
-
SPL UNCLASSIFIED SECTIONLister-V™ PRODUCT INFORMATION Lister-V (U.S. patent pending) capsules by oral administration. A specially formulated Medical Food product, consisting of a proprietary blend of amino acids and ...
-
DESCRIPTIONPRODUCT DESCRIPTION Primary Ingredients Lister-V consists of a proprietary blend of amino acids, cocoa, caffeine, cinnamon, and flavonoids in specific proportions. These ingredients fall into ...
-
CLINICAL PHARMACOLOGYCLINICAL PHARMACOLOGY Mechanism of Action Lister-V acts by activating white blood cell production of several types of white blood cells to combat and destroy viruses when they invade the body ...
-
INDICATIONS & USAGEINDICATIONS FOR USE Lister-V is intended for the clinical nutritional management of the metabolic processes associated with viral infections, including common cold and influenza (flu ...
-
CLINICAL STUDIESCLINICAL EXPERIENCE Administration of Lister-V has demonstrated significant functional improvements of influenza symptoms when used for the nutritional management of the metabolic processes ...
-
CONTRAINDICATIONSPRECAUTIONS AND CONTRAINDICATIONS Lister-V is contraindicated in an extremely small number of patients with hypersensitivity to any of the nutritional components of Lister-V.
-
ADVERSE REACTIONSADVERSE REACTIONS Oral supplementation with L-arginine at high doses up to 15 grams daily is generally well tolerated. The most common adverse reactions of higher doses — from 15 to 30 grams ...
-
DRUG INTERACTIONSDRUG INTERACTIONS Lister-V does not directly influence the pharmacokinetics of prescription drugs. Clinical experience has shown that administration of Lister-V may allow for lowering the dose of ...
-
OVERDOSAGEOVERDOSE There is a negligible risk of overdose with Lister-V as the total dosage of amino acids in a one month supply (60 capsules) is less than 25 grams. Overdose symptoms may include diarrhea ...
-
DOSAGE & ADMINISTRATIONDOSAGE AND ADMINISTRATION Recommended Administration For the nutritional management of the metabolic processes associated with viral infections. Lister-V can be administered to ameliorate the ...
-
HOW SUPPLIEDHOW SUPPLIED Lister-V is supplied in opaque white, size 0 capsules in bottles of 60 capsules. PHYSICIAN SUPERVISION Lister-V is a Medical Food product available by prescription only and must be ...
-
STORAGE AND HANDLINGSTORAGE Store at room temperature, 59-86OF (15-30OC). Protect from light and moisture.
-
PRINCIPAL DISPLAY PANELPHYSICIAN THERAPEUTICS LISTER-V Medical Food Rx only 60 Capsules Directions for use: Must be administered under physician supervision. For adults only. As a Medical Food, take two (2 ...
-
NONCLINICAL TOXICOLOGYFor the Dietary Management of Viral Infection. Three capsules four times daily or as directed by physician. See product label and insert. Lister-V Medical Food A Convenience Packed Medical Food ...
-
PRINCIPAL DISPLAY PANEL
 ...
... -
PRINCIPAL DISPLAY PANEL

-
INGREDIENTS AND APPEARANCEProduct Information