Label: ALLOPURINOL- allopurinol tablet
- NDC Code(s): 50090-7351-0, 50090-7351-1, 50090-7351-3
- Packager: A-S Medication Solutions
- This is a repackaged label.
- Source NDC Code(s): 31722-253
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 21, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
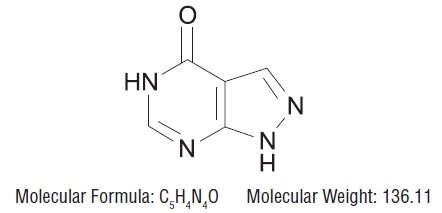
DESCRIPTIONAllopurinol, USP has the following structural formula: Allopurinol, USP is known chemically as 1,5-dihydro-4 H-pyrazolo [3, 4- d] pyrimidin-4-one. It is a xanthine oxidase inhibitor which is ...
-
CLINICAL PHARMACOLOGYAllopurinol acts on purine catabolism, without disrupting the biosynthesis of purines. It reduces the production of uric acid by inhibiting the biochemical reactions immediately preceding its ...
-
INDICATIONS & USAGETHIS IS NOT AN INNOCUOUS DRUG. IT IS NOT RECOMMENDED FOR THE TREATMENT OF ASYMPTOMATIC HYPERURICEMIA. Allopurinol tablets, USP reduces serum and urinary uric acid concentrations. Its use ...
-
CONTRAINDICATIONSPatients who have developed a severe reaction to allopurinol should not be restarted on the drug.
-
WARNINGSAllopurinol SHOULD BE DISCONTINUED AT THE FIRST APPEARANCE OF SKIN RASH OR OTHER SIGNS WHICH MAY INDICATE AN ALLERGIC REACTION. In some instances a skin rash may be followed by more severe ...
-
PRECAUTIONSGENERAL PRECAUTIONS - An increase in acute attacks of gout has been reported during the early stages of administration of allopurinol, even when normal or subnormal serum uric acid levels have ...
-
ADVERSE REACTIONSData upon which the following estimates of incidence of adverse reactions are made are derived from experiences reported in the literature, unpublished clinical trials and voluntary reports ...
-
OVERDOSAGEMassive overdosing or acute poisoning by allopurinol has not been reported. In mice, the 50% lethal dose (LD - 50) is 160 mg/kg given intraperitoneally (IP) with deaths delayed up to 5 ...
-
DOSAGE & ADMINISTRATIONThe dosage of allopurinol tablets, USP to accomplish full control of gout and to lower serum uric acid to normal or near-normal levels varies with the severity of the disease. The average is 200 ...
-
HOW SUPPLIEDProduct: 50090-7351 - NDC: 50090-7351-0 100 TABLET in a BOTTLE - NDC: 50090-7351-1 30 TABLET in a BOTTLE - NDC: 50090-7351-3 90 TABLET in a BOTTLE
-
Allopurinol

-
INGREDIENTS AND APPEARANCEProduct Information