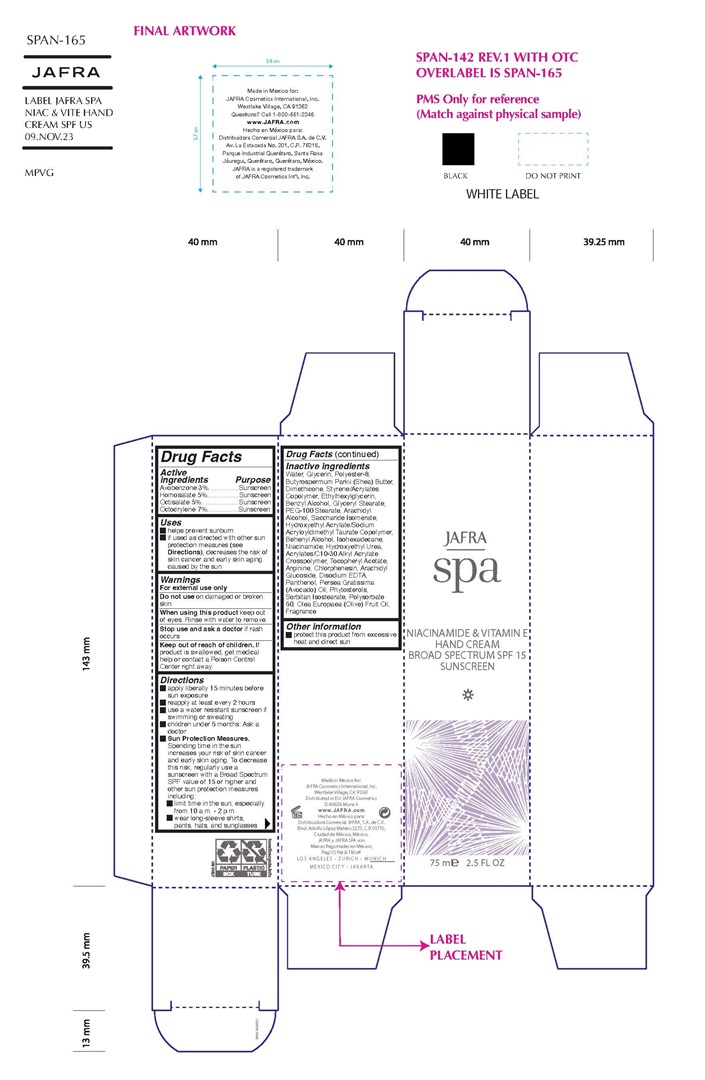
Label: SPA NIACINAMIDE AND VITAMIN E HAND CREAM BROAD SPECTRUM SPF 15- avobenzone, homosalate, octisalate, octocrylene cream
- NDC Code(s): 68828-003-01, 68828-003-02
- Packager: Jafra Cosmetics International Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warning
-
Direction
• Apply liberally 15 minutes before sun exposure
• Reapply at least every 2 hours
• Use a water resistant sunscreen if swimming or sweating
• Children under 6 months of age: Ask a doctor
• Sun Protection Measures.Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• limit your time in the sun, especially from 10 a.m. – 2 p.m.
• wear long-sleeved shirts, pants, hats, and sunglasses - KEEP OUT OF REACH OF CHILDREN
-
Inactive ingredients
Water, Glycerin, Polyester-8, Butyrospermum Parkii (Shea) Butter, Dimethicone, Styrene/Acrylates Copolymer, Ethylhexylglycerin, Benzyl Alcohol, Glyceryl Stearate, PEG-100 Stearate, Arachidyl Alcohol, Saccharide Isomerate, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Behenyl Alcohol, Isohexadecane, Niacinamide, Hydroxyethyl Urea, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Tocopheryl Acetate, Arginine, Chlorphenesin, Arachidyl Glucoside, Disodium EDTA, Panthenol, Persea Gratissima (Avocado) Oil, Phytosterols, Sorbitan Isostearate, Polysorbate 60, Olea Europaea (Olive) Fruit Oil, Fragrance
- Other information
- Product label
-
INGREDIENTS AND APPEARANCE
SPA NIACINAMIDE AND VITAMIN E HAND CREAM BROAD SPECTRUM SPF 15
avobenzone, homosalate, octisalate, octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68828-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 5 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) SHEA BUTTER (UNII: K49155WL9Y) DIMETHICONE (UNII: 92RU3N3Y1O) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) BENZYL ALCOHOL (UNII: LKG8494WBH) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) ARACHIDYL ALCOHOL (UNII: 1QR1QRA9BU) SACCHARIDE ISOMERATE (UNII: W8K377W98I) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) DOCOSANOL (UNII: 9G1OE216XY) ISOHEXADECANE (UNII: 918X1OUF1E) NIACINAMIDE (UNII: 25X51I8RD4) HYDROXYETHYL UREA (UNII: MBQ7DDQ7AR) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ARGININE (UNII: 94ZLA3W45F) CHLORPHENESIN (UNII: I670DAL4SZ) ARACHIDYL GLUCOSIDE (UNII: 6JVW35JOOJ) EDETATE DISODIUM (UNII: 7FLD91C86K) PANTHENOL (UNII: WV9CM0O67Z) AVOCADO OIL (UNII: 6VNO72PFC1) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) POLYSORBATE 60 (UNII: CAL22UVI4M) OLIVE OIL (UNII: 6UYK2W1W1E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68828-003-01 1 in 1 CARTON 06/01/2022 1 75 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:68828-003-02 1 in 1 CARTON 06/01/2022 2 7 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/01/2022 Labeler - Jafra Cosmetics International Inc (041676479) Registrant - Jafra Cosmetics International Inc (041676479) Establishment Name Address ID/FEI Business Operations Distribuidora Comercial Jafra, S.A. de C.V. 951612777 manufacture(68828-003)