Label: PENTAMIDINE ISETHIONATE injection, powder, lyophilized, for solution
- NDC Code(s): 39822-3050-2
- Packager: XGen Pharmaceuticals DJB, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONFor Injection Only
-
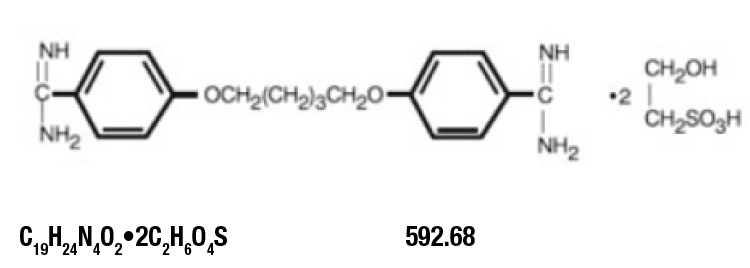
DESCRIPTION
Pentamidine isethionate for injection, an anti-protozoal agent, is a sterile, nonpyrogenic, lyophilized product. After reconstitution, it should be administered by intramuscular (IM) or ...
-
CLINICAL PHARMACOLOGY
Pentamidine isethionate, an aromatic diamidine, is known to have activity against - Pneumocystis carinii. The mode of action of pentamidine is not fully understood. In vitro studies indicate ...
-
INDICATIONS AND USAGE
Pentamidine isethionate for injection is indicated for the treatment of pneumonia due to - Pneumocystis carinii.
-
CONTRAINDICATIONS
Contraindicated in patients with a history of hypersensitivity to pentamidine isethionate.
-
WARNINGS
Fatalities due to severe hypotension, hypoglycemia, acute pancreatitis and cardiac arrhythmias have been reported in patients treated with pentamidine isethionate, both by the IM and IV routes ...
-
PRECAUTIONS
General - Pentamidine isethionate should be used with caution in patients with hypertension, hypotension, ventricular tachycardia, hypoglycemia, hyperglycemia, hypocalcemia, pancreatitis ...
-
ADVERSE REACTIONS
CAUTION: Fatalities due to severe hypotension, hypoglycemia, acute pancreatitis and cardiac arrhythmias have been reported in patients treated with pentamidine isethionate, both by the IM and IV ...
-
OVERDOSAGE
A 17 month old infant inadvertently received 1600 mg of intravenous pentamidine isethionate which was followed by renal and hepatic function impairment, hypotension and cardiopulmonary arrest ...
-
DOSAGE AND ADMINISTRATION
CAUTION:DO NOT USE SODIUM CHLORIDE INJECTION, USP FOR INITIAL RECONSTITUTION BECAUSE PRECIPITATION WILL OCCUR. Pentamidine isethionate should be administered IM or IV only. The recommended ...
-
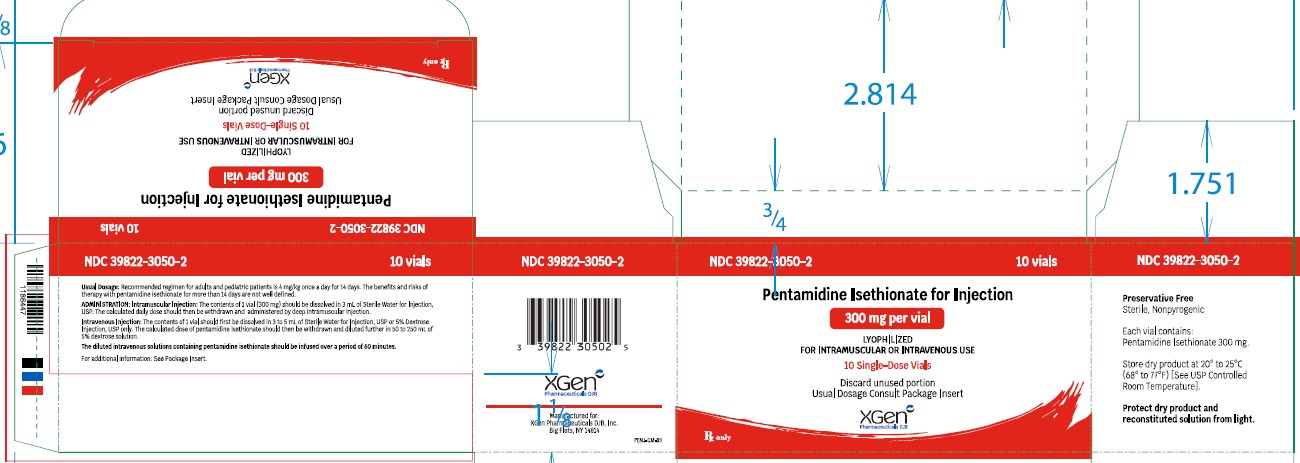
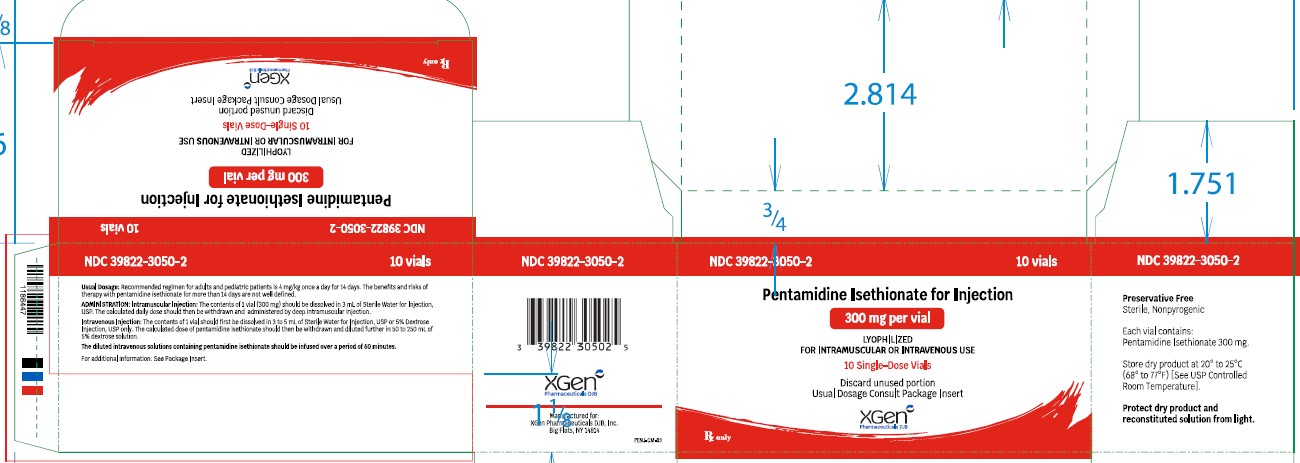
HOW SUPPLIED
NDC No. Pentamidine isethionate for injection 300 mg, lyophilized product in Single-dose Vials (NDC 39822-3050-1), packages of 10 (NDC 39822-3050-2). Store dry product at 20° to 25°C ...
-
REFERENCE
Watts RG; Conte JE, Jr.; Zurlinden E; Waldo FB: Effect of charcoal hemoperfusion on clearance of pentamidine isethionate after accidental overdose. J Toxicol Clin Toxicol ...
-
[PACKAGE LABEL.PRINCIPAL DISPLAY PANEL]
PACKAGE LABEL - PRINCIPAL DISPLAY – PENTAMIDINE ISETHIONATE FOR INJECTION 300 mg Single Dose Vial Label - NDC 39822-3050-1 - Pentamidine Isethionate for Injection) 300 mg - Lyophilized - For IM or IV ...
-
INGREDIENTS AND APPEARANCEProduct Information