Label: CALCIUM ACETATE capsule
- NDC Code(s): 0054-0088-13, 0054-0088-26
- Packager: Hikma Pharmaceuticals USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 17, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Calcium Acetate Capsules safely and effectively. See full prescribing information for Calcium Acetate Capsules. Calcium Acetate ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGECalcium acetate is a phosphate binder indicated to reduce serum phosphorus in patients with end stage renal disease (ESRD).
-
2 DOSAGE AND ADMINISTRATIONThe recommended initial dose of calcium acetate for the adult dialysis patient is 2 capsules with each meal. Increase the dose gradually to lower serum phosphorus levels to the target range, as ...
-
3 DOSAGE FORMS AND STRENGTHSCapsule: 667 mg calcium acetate capsule.
-
4 CONTRAINDICATIONSPatients with hypercalcemia.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypercalcemia - Patients with end stage renal disease may develop hypercalcemia when treated with calcium, including calcium acetate. Avoid the use of calcium supplements, including calcium ...
-
6 ADVERSE REACTIONSHypercalcemia is discussed elsewhere [see Warnings and Precautions (5.1)]. 6.1 Clinical Trial Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction ...
-
7 DRUG INTERACTIONSThe drug interaction of calcium acetate is characterized by the potential of calcium to bind to drugs with anionic functions (e.g., carboxyl, and hydroxyl groups). Calcium acetate may decrease the ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Category C: Calcium acetate capsules contains calcium acetate. Animal reproduction studies have not been conducted with calcium acetate, and there are no adequate and ...
-
10 OVERDOSAGEAdministration of calcium acetate in excess of the appropriate daily dosage may result in hypercalcemia [see Warnings and Precautions (5.1)].
-
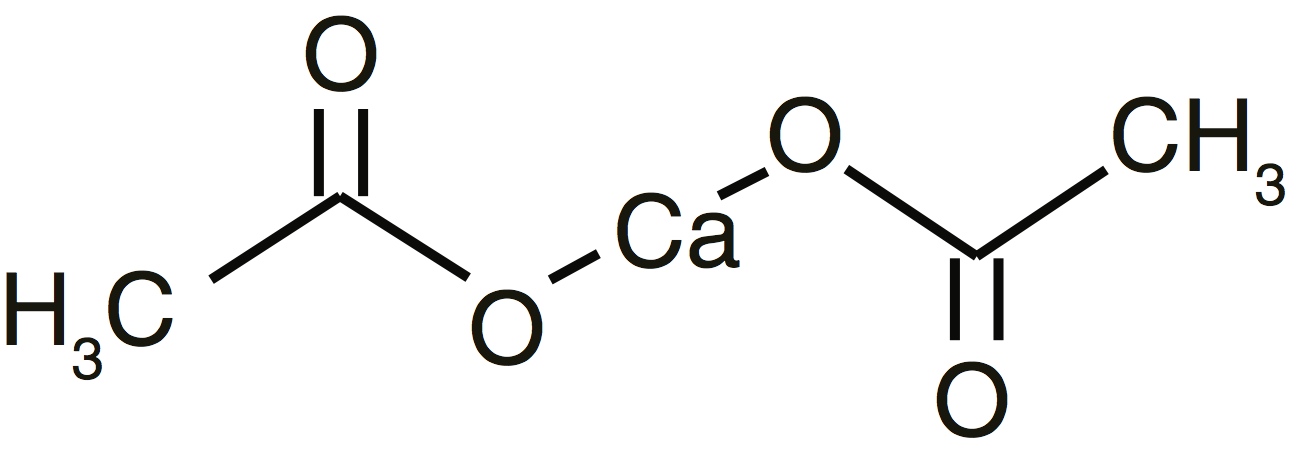
11 DESCRIPTIONCalcium acetate acts as a phosphate binder. Its chemical name is calcium acetate. Its molecular formula is C4H6CaO4, and its molecular weight is 158.17. Its structural formula is: Each white ...
-
12 CLINICAL PHARMACOLOGYPatients with ESRD retain phosphorus and can develop hyperphosphatemia. High serum phosphorus can precipitate serum calcium resulting in ectopic calcification. Hyperphosphatemia also plays a role ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No carcinogenicity, mutagenicity, or fertility studies have been conducted with calcium acetate.
-
14 CLINICAL STUDIESEffectiveness of calcium acetate in decreasing serum phosphorus has been demonstrated in two studies of the calcium acetate solid oral dosage form. Ninety-one patients with end-stage renal ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGCalcium Acetate Capsules, USP - 667 mg capsule is supplied as a white opaque/blue opaque capsule, imprinted with “54 215” on the cap and body. NDC 0054-0088-13: Bottle of 30 Capsules - NDC ...
-
17 PATIENT COUNSELING INFORMATIONInform patients to take calcium acetate capsules with meals, adhere to their prescribed diets, and avoid the use of calcium supplements including nonprescription antacids. Inform the patients ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL

-
INGREDIENTS AND APPEARANCEProduct Information