Label: XIIDRA- lifitegrast solution/ drops
- NDC Code(s): 0078-0911-05, 0078-0911-12, 0078-0911-94, 0078-0911-95
- Packager: Novartis Pharmaceuticals Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use XIIDRA safely and effectively. See full prescribing information for XIIDRA. XIIDRA® (lifitegrast ophthalmic solution), for topical ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEXiidra® (lifitegrast ophthalmic solution) 5% is indicated for the treatment of the signs and symptoms of dry eye disease (DED).
-
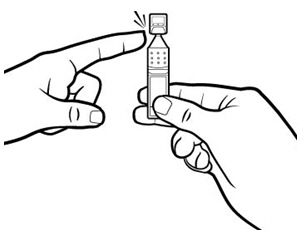
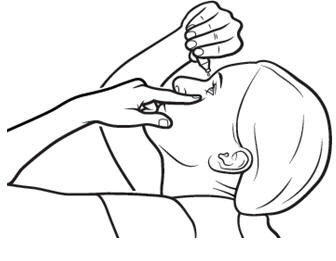
2 DOSAGE AND ADMINISTRATIONInstill one drop of Xiidra twice daily (approximately 12 hours apart) into each eye using a single-use container. Discard the single-use container immediately after using in each eye. Contact ...
-
3 DOSAGE FORMS AND STRENGTHSOphthalmic solution containing lifitegrast 50 mg/mL (5%).
-
4 CONTRAINDICATIONSXiidra is contraindicated in patients with known hypersensitivity to lifitegrast or to any of the other ingredients in the formulation [see Adverse Reactions (6.2)].
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described elsewhere in the labeling: Hypersensitivity [see Contraindications (4)] 6.1 Clinical Trials Experience - Because clinical trials are ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on Xiidra use in pregnant women to inform any drug-associated risks. Intravenous (IV) administration of lifitegrast to pregnant ...
-
11 DESCRIPTIONThe chemical name for lifitegrast is (S)-2-(2-(benzofuran-6-carbonyl)-5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carboxamido)-3-(3-(methylsulfonyl)phenyl)propanoic acid. The molecular formula ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Lifitegrast binds to the integrin LFA-1, a cell surface protein found on leukocytes and blocks the interaction of LFA-1 with its cognate ligand intercellular ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Animal studies have not been conducted to determine the carcinogenic potential of ...
-
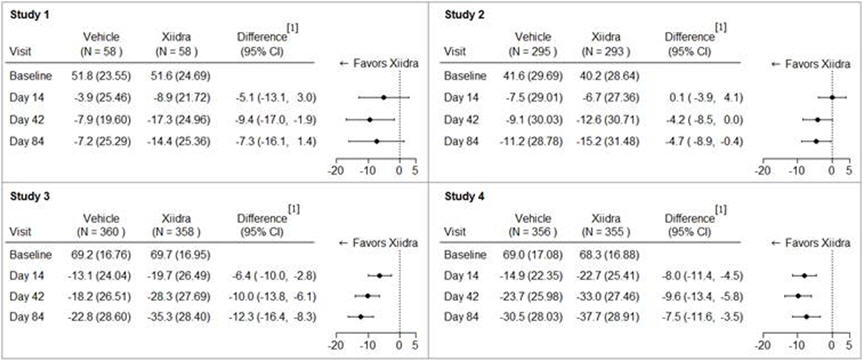
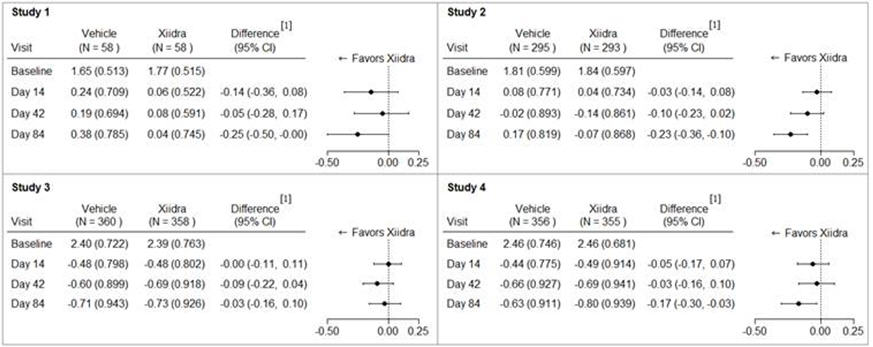
14 CLINICAL STUDIESThe safety and efficacy of lifitegrast for the treatment of DED were assessed in a total of 1181 patients (1067 of which received lifitegrast 5%) in four 12-week, randomized, multi-center ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGXiidra (lifitegrast ophthalmic solution) 5% (50 mg/mL) is supplied in a foil pouch containing 5 low-density polyethylene 0.2 mL single-use containers. Carton of 60 single-use ...
-
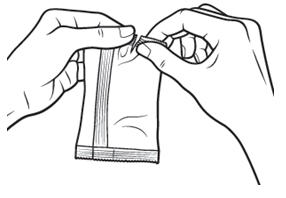
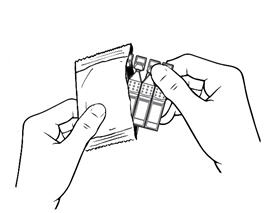
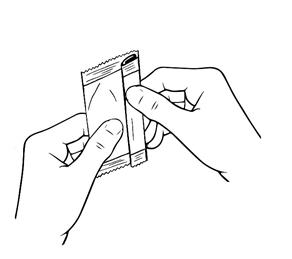
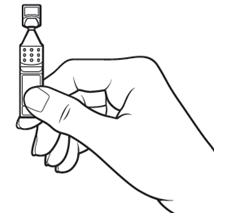
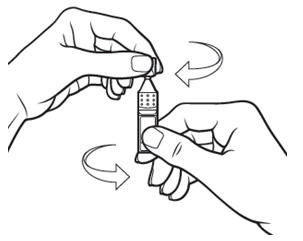
17 PATIENT COUNSELING INFORMATIONAdvise patients to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Handling the Single-use Container - Advise patients not to touch the tip of the single-use ...
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration.Revised: June 2020 - PATIENT INFORMATION - XIIDRA® (ZYE-druh) (lifitegrast ophthalmic solution ...
-
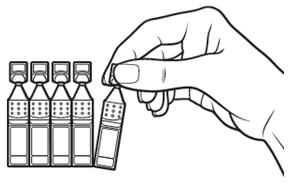
INSTRUCTIONS FOR USEThis Instructions for Use has been approved by the U.S. Food and Drug Administration.Revised: June 2020 - INSTRUCTIONS FOR USE - XIIDRA® [ZYE-druh] (lifitegrast ophthalmic ...
-
PRINCIPAL DISPLAY PANELNDC 0078-0911-12 - Rx Only - 60 Single-Use - Containers: 12 pouches x 5 single-use - containers (0.2 mL each) xiidra® (lifitegrast - ophthalmic solution) 5% NOVARTIS
-
INGREDIENTS AND APPEARANCEProduct Information