Label: BUSCOPAN- n-butylscopolammonium bromide injection
- NDC Code(s): 0010-3701-01
- Packager: Boehringer Ingelheim Animal Health USA Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated March 27, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
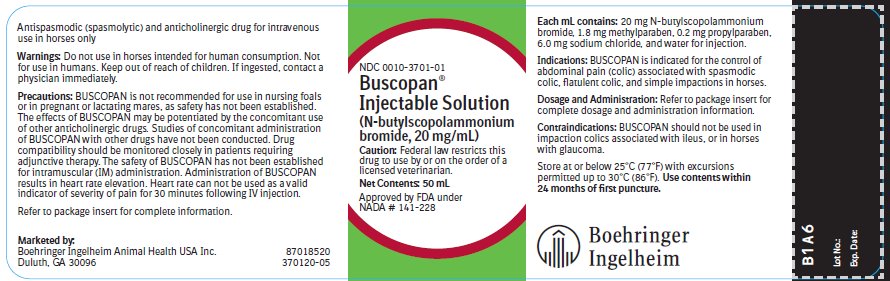
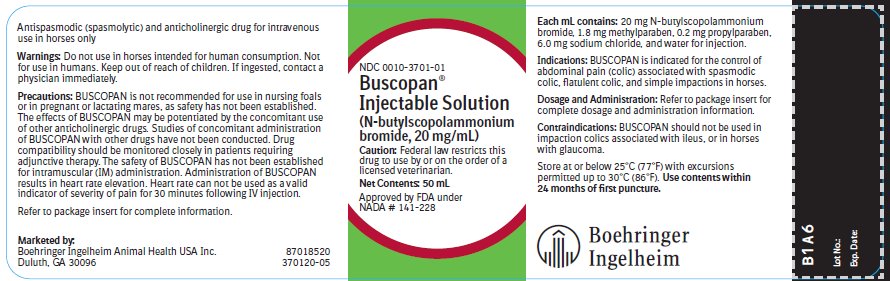
SPL UNCLASSIFIED SECTIONApproved by FDA under NADA # 141-228 - Antispasmodic (spasmolytic) and anticholinergic drug for intravenous use in horses only.
-
Caution:Federal law restricts this drug to use by or on the order of a licensed veterinarian.
-
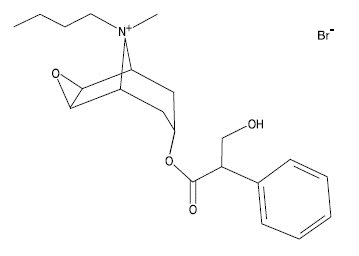
Description:BUSCOPAN Injectable Solution is an antispasmodic (spasmolytic) and anticholinergic drug which suppresses spasms of the digestive system. The chemical name for the active constituent of BUSCOPAN ...
-
Indications:BUSCOPAN is indicated for the control of abdominal pain (colic) associated with spasmodic colic, flatulent colic, and simple impactions in horses.
-
Dosage and Administration:Administer a single injection of 0.3 mg/kg body weight (0.14 mg/lb), slowly IV. This is equivalent to 30 mg N-butylscopolammonium bromide per 100 kg (220 pounds) bodyweight or 1.5 mL of BUSCOPAN ...
-
Contraindications:BUSCOPAN should not be used in impaction colics associated with ileus, or in horses with glaucoma.
-
Warnings:Do not use in horses intended for human consumption. Not for use in humans. Keep out of reach of children. If ingested, contact a physician immediately.
-
Precautions:BUSCOPAN is not recommended for use in nursing foals or in pregnant or lactating mares, as safety has not been established. The effects of BUSCOPAN may be potentiated by the concomitant use of ...
-
Adverse Reactions:Transient tachycardia and decreased borborygmal sounds lasted approximately 30 minutes following administration. Transient pupillary dilation may also be observed. To report suspected adverse ...
-
Clinical Pharmacology:The spasmolytic action of BUSCOPAN is based on anticholinergic effects resulting from competitive inhibition of parasympathetic activation (via muscarinic receptors) of smooth muscle cells.1 The ...
-
Pharmacokinetics: Following single IV administration of 14C-Buscopan (0.4 mg/kg, side chain labeled) in 3 horses, the major route of elimination of total radioactivity was via urine and feces almost equally. The ...
-
Effectiveness: A multi-centered, field study was conducted to establish the clinical effectiveness of BUSCOPAN (0.3 mg/kg body weight) for the control of abdominal pain (colic) associated with spasmodic ...
-
Animal Safety: Target animal safety was evaluated in several studies, including dose tolerance, target animal safety, hemodynamics, and field safety. There were no signs of toxicity or adverse reactions. The ...
-
Storage:Store at or below 25°C (77°F) with excursions permitted up to 30°C (86°F). Use contents within 24 months of first puncture.
-
How Supplied:BUSCOPAN Injectable Solution is supplied in 50 mL multi-dose vials containing 20 mg N-butylscopolammonium bromide per mL. NDC 0010-3701-01 - 50 mL vial
-
References:1Roelvink, M.E.J., et al. 1991. Analgesic and spasmolytic effects of dipyrone, hyoscine-N-butylbromide and a combination of the two in ponies. Veterinary Record 129:379-380
-
SPL UNCLASSIFIED SECTIONMarketed by: Boehringer Ingelheim Animal Health USA Inc. Duluth, GA 30096 - BUSCOPAN is a registered trademark of Boehringer Ingelheim Pharma GmbH & Co. KG, used under license. ©2019 Boehringer ...
-
50 mL Container Label NDC 0010-3701-01 - Busopan® Injectable Solution - (N-butylscopolammonium bromide, 20 mg/mL) Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian. Net ...
-
50 mL Display Carton NDC 0010-3701-01 - Busopan® Injectable Solution - (N-butylscopolammonium bromide, 20 mg/mL) Caution: Federal law restricts this drug to use by or on the order of a licensed ...
-
INGREDIENTS AND APPEARANCEProduct Information