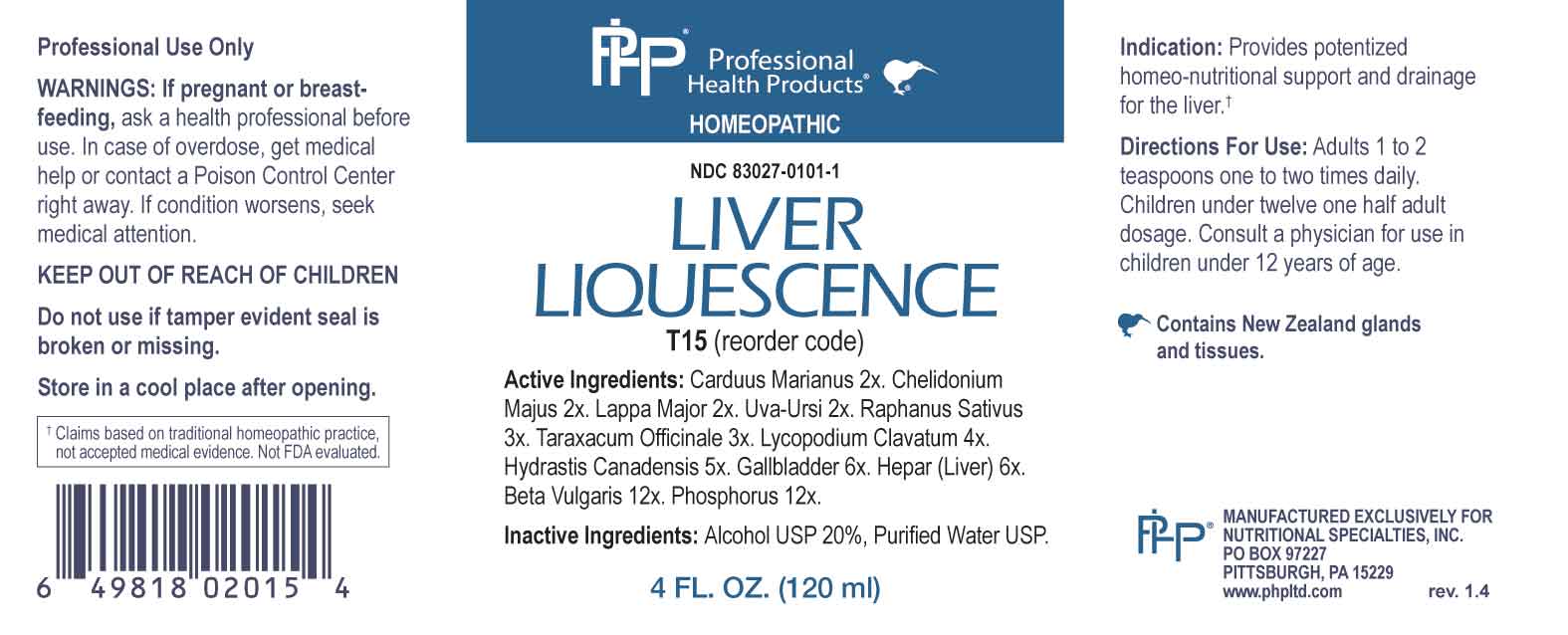
Label: LIVER LIQUESCENCE (carduus marianus, chelidonium majus, lappa major, uva ursi, raphanus sativus, taraxacum officinale, lycopodium clavatum, hydrastis canadensis, gallbladder (bovine), hepar- bovine, beta vulgaris, phosphorus liquid
- NDC Code(s): 83027-0101-1
- Packager: Nutritional Specialties, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated September 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS:
- PURPOSE:
-
WARNINGS:
Professional Use Only
If pregnant or breast-feeding, ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away.
If condition worsens, seek medical attention.
KEEP OUT OF REACH OF CHILDREN
Do not use if tamper evident seal is broken or missing.
Store in a cool place after opening
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- INDICATIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
LIVER LIQUESCENCE
carduus marianus, chelidonium majus, lappa major, uva ursi, raphanus sativus, taraxacum officinale, lycopodium clavatum, hydrastis canadensis, gallbladder (bovine), hepar (bovine), beta vulgaris, phosphorus liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83027-0101 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MILK THISTLE (UNII: U946SH95EE) (MILK THISTLE - UNII:U946SH95EE) MILK THISTLE 2 [hp_X] in 1 mL CHELIDONIUM MAJUS WHOLE (UNII: 7E889U5RNN) (CHELIDONIUM MAJUS - UNII:7E889U5RNN) CHELIDONIUM MAJUS WHOLE 2 [hp_X] in 1 mL ARCTIUM LAPPA ROOT (UNII: 597E9BI3Z3) (ARCTIUM LAPPA ROOT - UNII:597E9BI3Z3) ARCTIUM LAPPA ROOT 2 [hp_X] in 1 mL ARCTOSTAPHYLOS UVA-URSI LEAF (UNII: 3M5V3D1X36) (ARCTOSTAPHYLOS UVA-URSI LEAF - UNII:3M5V3D1X36) ARCTOSTAPHYLOS UVA-URSI LEAF 2 [hp_X] in 1 mL RADISH (UNII: EM5RP35463) (RADISH - UNII:EM5RP35463) RADISH 3 [hp_X] in 1 mL TARAXACUM OFFICINALE (UNII: 39981FM375) (TARAXACUM OFFICINALE - UNII:39981FM375) TARAXACUM OFFICINALE 3 [hp_X] in 1 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 4 [hp_X] in 1 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 5 [hp_X] in 1 mL BOS TAURUS GALLBLADDER (UNII: 9901V1E867) (BOS TAURUS GALLBLADDER - UNII:9901V1E867) BOS TAURUS GALLBLADDER 6 [hp_X] in 1 mL HEPARIN, BOVINE (UNII: P776JQ4R2F) (HEPARIN, BOVINE - UNII:P776JQ4R2F) HEPARIN, BOVINE 6 [hp_X] in 1 mL BETA VULGARIS WHOLE (UNII: 4G174V5051) (BETA VULGARIS - UNII:4G174V5051) BETA VULGARIS WHOLE 12 [hp_X] in 1 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 12 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83027-0101-1 120 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 09/11/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/11/2023 Labeler - Nutritional Specialties, Inc. (032744609)