Label: ACTIVICE- menthol liquid
- NDC Code(s): 53329-992-69
- Packager: Medline Industries, LP
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
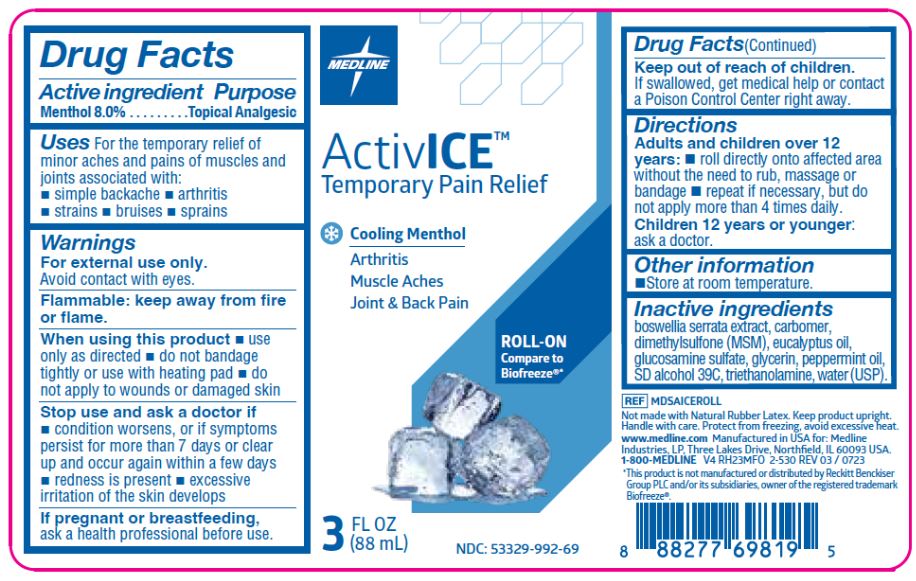
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external use only.
Avoid contact with eyes.
Flammable: keep away from fire
or flame.When using this product
- use only as directed
- do not bandage tightly or use with heating pad
- do not apply to wounds or damaged skin
- Directions
- Other information
- Inactive Ingredients
- Manufacturing Information
- Package Labels
-
INGREDIENTS AND APPEARANCE
ACTIVICE
menthol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53329-992 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 80 g in 1000 g Inactive Ingredients Ingredient Name Strength CARBOMER 940 (UNII: 4Q93RCW27E) ALCOHOL (UNII: 3K9958V90M) GLUCOSAMINE SULFATE (UNII: 1FW7WLR731) TROLAMINE (UNII: 9O3K93S3TK) GLYCERIN (UNII: PDC6A3C0OX) BOSWELLIA SERRATA RESIN OIL (UNII: 5T1XCE6K8K) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) PEPPERMINT OIL (UNII: AV092KU4JH) WATER (UNII: 059QF0KO0R) EUCALYPTUS OIL (UNII: 2R04ONI662) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53329-992-69 85 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/04/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 03/04/2019 Labeler - Medline Industries, LP (025460908) Registrant - Medline Industries, LP (025460908)