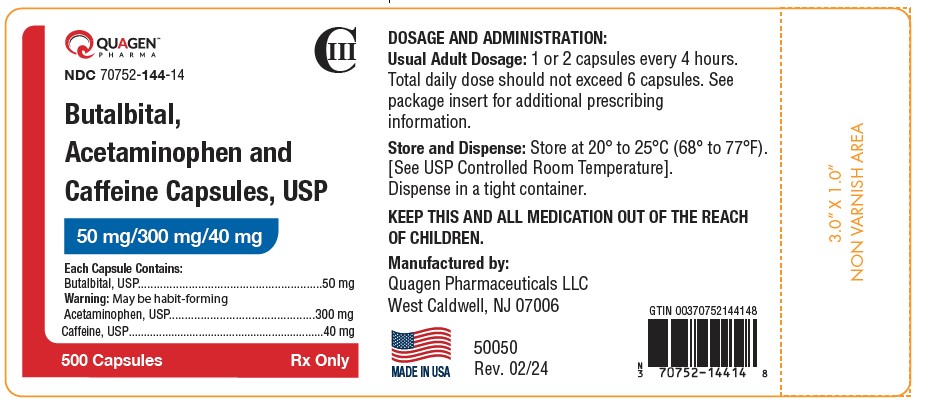
Label: BUTALBITAL, ACETAMINOPHEN AND CAFFEINE capsule
- NDC Code(s): 70752-144-10, 70752-144-14
- Packager: QUAGEN PHARMACEUTICALS LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIII
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING
HEPATOTOXICITY
Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4000 milligrams per day, and often involve more than one acetaminophen containing product.
Close -
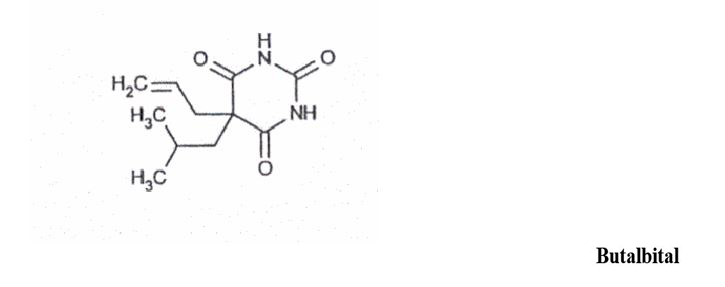
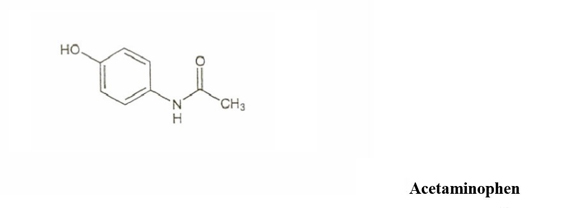
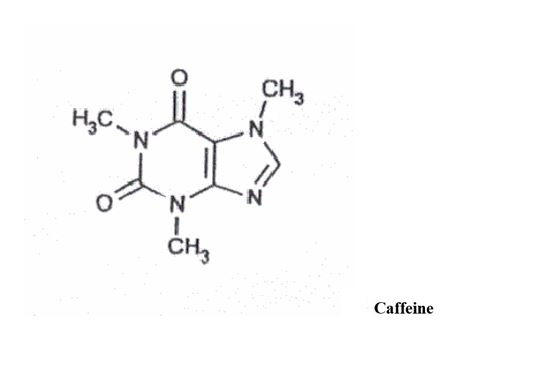
DESCRIPTIONButalbital, Acetaminophen and Caffeine Capsules USP are supplied in hard-gelatin capsule form for oral administration. Each capsule contains the following active ingredients: butalbital ...
-
CLINICAL PHARMACOLOGYThis combination drug product is intended as a treatment for tension headache. It consists of a fixed combination of butalbital, acetaminophen, and caffeine. The role each component plays in the ...
-
INDICATIONS AND USAGEButalbital, Acetaminophen and Caffeine capsules are indicated for the relief of the symptom complex of tension (or muscle contraction) headache. Evidence supporting the efficacy and safety of this ...
-
CONTRAINDICATIONSThis product is contraindicated under the following conditions: Hypersensitivity or intolerance to any component of this product. Patients with porphyria.
-
WARNINGSButalbital is habit-forming and potentially abusable. Consequently, the extended use of this product is not recommended. Hepatotoxicity - Acetaminophen has been associated with cases of acute ...
-
PRECAUTIONSGeneral - Butalbital, acetaminophen, and caffeine capsules should be prescribed with caution in certain special-risk patients, such as the elderly or debilitated, and those with severe impairment ...
-
ADVERSE REACTIONSFrequently Observed - The most frequently reported adverse reactions are drowsiness, lightheadedness, dizziness, sedation, shortness of breath, nausea, vomiting, abdominal pain, and intoxicated ...
-
DRUG ABUSE AND DEPENDENCEAbuse and Dependence - Butalbital - Barbiturates may be habit-forming:Tolerance, psychological dependence, and physical dependence may occur especially following prolonged use of high doses of ...
-
OVERDOSAGEFollowing an acute overdosage of butalbital, acetaminophen, and caffeine, toxicity may result from the barbiturate or the acetaminophen. Toxicity due to caffeine is less likely, due to the ...
-
DOSAGE AND ADMINISTRATIONOne or 2 capsules every 4 hours as needed. Total daily dosage should not exceed 6 capsules. Extended and repeated use of this product is not recommended because of the potential for physical ...
-
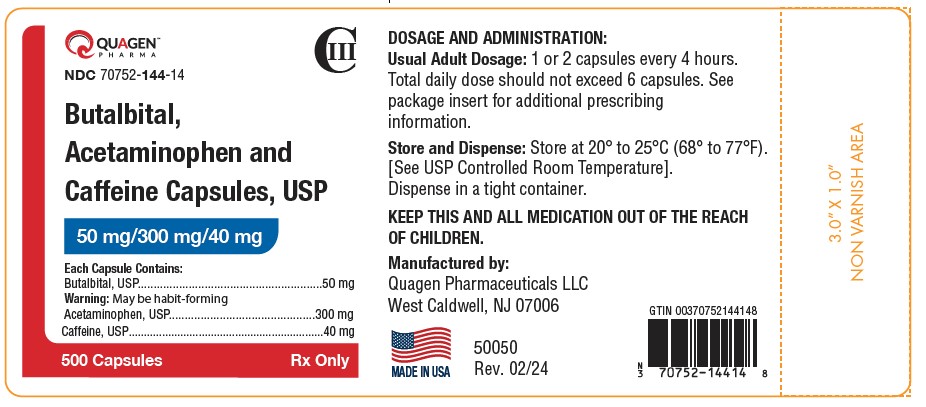
HOW SUPPLIEDButalbital, Acetaminophen, and Caffeine Capsules USP, 50 mg/300 mg/40 mg - Containing: 50 mg butalbital (WARNING: May be habit-forming.), 300 mg acetaminophen, and 40 mg caffeine. Available as ...
-
SPL UNCLASSIFIED SECTIONRx only - Manufactured by: Quagen Pharmaceuticals LLC - West Caldwell, NJ 07006 - 52018 - Rev. 02/24
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - NDC 70752-144-10 - Butalbital, Acetaminophen - and Caffeine Capsules USP - 50 mg/300 mg/40 mg - 100 Capsules - Rx only - PRINCIPAL DISPLAY PANEL - NDC ...
-
INGREDIENTS AND APPEARANCEProduct Information