General
-
Hepatic Impairment
-
Patients with hepatic impairment metabolize metronidazole slowly, with resultant accumulation of metronidazole and increase the plasma concentrations. Reduce the ...
General
Hepatic Impairment
Patients with hepatic impairment metabolize metronidazole slowly, with resultant accumulation of metronidazole and increase the plasma concentrations. Reduce the dose of metronidazole injection by 50% in patients with severe hepatic impairment (Child-Pugh C). For patients with mild to moderate hepatic impairment, no dosage adjustment is needed but these patients should be monitored for metronidazole associated adverse events (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION).
Patients with severe hepatic encephalopathy metabolize metronidazole slowly, with resultant accumulation of metronidazole. This may cause exacerbation of CNS adverse effects. Reduce the dose of metronidazole injection as necessary.
Renal Impairment
For patients with mild to moderate renal impairment dose adjustment is not considered necessary as elimination half-life is not significantly altered. In patients with severe renal impairment or end stage of renal disease, metronidazole and metronidazole metabolites may accumulate significantly because of reduced urinary excretion in those patients. Monitoring for metronidazole associated adverse events is recommended when metronidazole is administered in patients with severe renal impairment or end stage of renal disease who are not undergoing hemodialysis (see CLINICAL PHARMACOLOGY).
Hemodialysis removes significant amounts of metronidazole and its metabolites from systemic circulation. Therefore, supplementation of metronidazole following a hemodialysis session may be necessary.
Patients receiving peritoneal dialysis should be monitored for signs of toxicity due to the potential accumulation of metronidazole metabolites.
Fungal Superinfections
Known or previously unrecognized candidiasis may present more prominent symptoms during therapy with metronidazole injection and requires treatment with a candicidal agent.
Use in Patients with Blood Dyscrasias
Metronidazole is a nitroimidazole and should be used with care in patients with evidence of or history of blood dyscrasia. Agranulocytosis, leukopenia and neutropenia have been associated with metronidazole administration. Monitor complete blood count in these patients.
Monitoring for Leukopenia
Monitoring of complete blood count (CBC) is recommended before, during and after prolonged or repeated courses of metronidazole therapy.
Sodium Retention
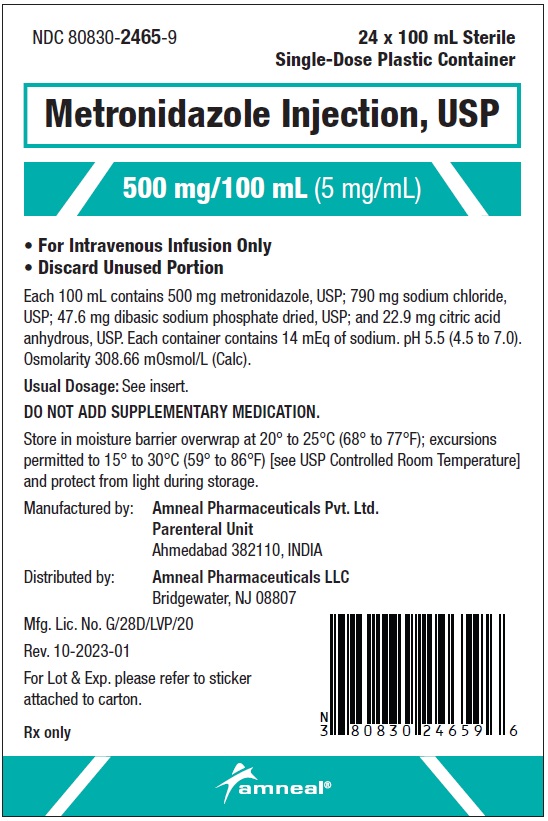
Metronidazole injection contains 790 mg of sodium per 100 mL. Care should be taken when administering metronidazole injection to patients receiving a controlled sodium diet or corticosteroids or to patients predisposed to edema.
Drug-Resistant Bacteria
Prescribing metronidazole injection in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Information for Patients
Discontinue consumption of alcoholic beverages or products containing propylene glycol while taking metronidazole injection and for at least three days afterward because abdominal cramps, nausea, vomiting, headaches, and flushing may occur (see CONTRAINDICATIONS, PRECAUTIONS-Drug Interactions).
-
Treatment of Bacterial Infections
Patients should be counseled that antibacterial drugs including metronidazole injection should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When metronidazole injection is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by metronidazole injection or other antibacterial drugs in the future.
-
Severe Cutaneous Adverse Reactions
Advise patients that Metronidazole Injection may increase the risk of serious and sometimes fatal dermatologic reactions, including toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome (SJS), and drug reaction with eosinophilia and systemic symptoms (DRESS). Instruct the patient to be alert for skin rash, blisters, fever or other signs and symptoms of these hypersensitivity reactions. Advise patients to stop Metronidazole Injection immediately if they develop any type of rash and seek medical attention.
Drug Interactions
Psychotic reactions and confusion have been reported in alcoholic patients who are using metronidazole and disulfiram concurrently. Do not administer metronidazole injection to patients who have taken disulfiram within the last two weeks (see CONTRAINDICATIONS).
Abdominal cramps, nausea, vomiting, headaches, tachycardia and flushing may occur if alcoholic beverages or products containing propylene glycol are consumed during or following metronidazole therapy. Discontinue consumption of alcohol or products containing propylene glycol before, during and up to 72 hours after therapy with metronidazole injection (see CONTRAINDICATIONS).
-
Warfarin and other Oral Anticoagulants
Metronidazole has been reported to potentiate the anticoagulant effect of warfarin and other oral coumarin anticoagulants, resulting in a prolongation of prothrombin time and increased risk of hemorrhages. When metronidazole injection is prescribed for patients on this type of anticoagulant therapy, prothrombin time and international normalized ratio (INR) should be carefully monitored and their anticoagulant dose adjusted accordingly. Monitor patients for signs and symptoms of bleeding.
In patients stabilized on relatively high doses of lithium, short-term metronidazole therapy has been associated with elevation of serum lithium and, in a few cases, signs of lithium toxicity. Lithium toxicity may lead to renal damage. Frequent monitoring of serum lithium and serum creatinine levels is necessary.
Metronidazole has been reported to increase plasma concentrations of busulfan, which can result in an increased risk for serious busulfan toxicity such as sinusoidal obstruction syndrome, gastrointestinal mucositis and hepatic veno-occlusive disease. Metronidazole injection should not be administered concomitantly with busulfan unless the benefit outweighs the risk. If no therapeutic alternatives to metronidazole are available, and concomitant administration with busulfan is medically needed, frequent monitoring of busulfan plasma concentration should be performed and the busulfan dose should be adjusted accordingly.
-
Drugs that Inhibit CYP450 Enzymes
The simultaneous administration of drugs that decrease microsomal liver enzyme activity, such as cimetidine, may decrease metabolism and reduce plasma clearance of metronidazole which may result in metronidazole toxicity.
-
Drugs that Induce CYP450 Enzymes
The simultaneous administration of drugs that induce microsomal liver enzyme activity, such as phenytoin or phenobarbital, may accelerate the elimination of metronidazole and therefore decrease its efficacy.
-
Cytochrome P450 3A4 (CYP3A4) substrates
Concomitant use of metronidazole injection and CYP3A4 substrates (e.g., amiodarone, tacrolimus, cyclosporine, carbamazepine, phenytoin and quinidine) may increase respective CYP3A4-substrate plasma levels. Monitoring of plasma concentrations of CYP3A4 substrates may be necessary.
Metronidazole injection decreases the clearance of 5-fluorouracil and may therefore cause 5-fluorouracil toxicity.
Metronidazole injection may potentiate the effects of vecuronium.
-
Drugs that Prolong the QT interval
QT prolongation has been reported, particularly when metronidazole was administered with drugs with the potential for prolonging the QT interval.
-
Drug/Laboratory Test Interactions
Metronidazole may interfere with certain types of determinations of serum chemistry values, such as aspartate aminotransferase (AST, SGOT), alanine aminotransferase (ALT, SGPT), lactate dehydrogenase (LDH), triglycerides and glucose hexokinase. Metronidazole causes an increase in ultraviolet absorbance at 340 nm resulting in falsely decreased values.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Tumors affecting the liver, lung, mammary and lymphatic tissues have been detected in several studies of metronidazole in rats and mice, but not hamsters.
Pulmonary tumors have been observed in all six reported studies in the mouse, including one study in which the animals were dosed on an intermittent schedule (administration during every fourth week only). Malignant tumors were increased in male mice treated at approximately 1,500 mg/m2 (similar to the maximum recommended daily dose, based on body surface area comparisons). Malignant lymphomas and pulmonary neoplasms were also increased with lifetime feeding of the drug to mice. Mammary and hepatic tumors were increased among female rats administered oral metronidazole compared to concurrent controls. Two lifetime tumorigenicity studies in hamsters have been performed and reported to be negative.
Metronidazole has shown mutagenic activity in in vitro assay systems including the Ames test. Studies in mammals in vivo have failed to demonstrate a potential for genetic damage.
Metronidazole failed to produce any adverse effects on fertility or testicular function in male rats at doses up to 400 mg/kg/day (approximately 2 times the maximum recommended daily dose based on body surface area comparison) for 28 days. However, rats treated at the same dose for 6 weeks, or longer were infertile and showed severe degeneration of the seminiferous epithelium in the testes as well as marked decreases in testicular spermatid counts and epididymal sperm counts. Fertility was restored in most rats after an eight week, drug-free recovery period.
Fertility studies have been performed in male mice at doses up to six times the maximum recommended human dose based on mg/m2 and have revealed no evidence of impaired fertility. However, metronidazole was associated with reversible adverse effects on the male reproductive system (significantly decreased testes and epididymides weight, decreased sperm viability, and increased the incidence of abnormal sperm).
Pregnancy
Teratogenic Effects
There are no adequate and well-controlled studies of metronidazole injection in pregnant women. There are published data from case-control studies, cohort studies and 2 meta-analyses that include more than 5,000 pregnant women who used metronidazole during pregnancy. Many studies included first trimester exposures. One study showed an increased risk of cleft lip, with or without cleft palate, in infants exposed to metronidazole in utero; however, these findings were not confirmed. In addition, more than ten randomized, placebo-controlled clinical trials enrolled more than 5,000 pregnant women to assess the use of antibiotic treatment (including metronidazole) for bacterial vaginosis on the incidence of preterm delivery. Most studies did not show an increased risk for congenital anomalies or other adverse fetal outcomes following metronidazole exposure during pregnancy. Three studies conducted to assess the risk of infant cancer following metronidazole exposure during pregnancy did not show an increased risk; however, the ability of these studies to detect such a signal was limited.
Metronidazole crosses the placental barrier and its effects on the human fetal organogenesis are not known. Reproduction studies have been performed in rats, rabbits and mice at doses similar to the maximum recommended daily dose based on body surface area comparisons. There was no evidence of harm to the fetus due to metronidazole.
Healthcare provider should carefully consider the potential risks and benefits for each specific patient before prescribing metronidazole injection.
Nursing Mothers
Metronidazole is present in human milk at concentrations similar to maternal serum levels, and infant serum levels can be close to or comparable to infant therapeutic levels. Because of the potential for tumorigenicity shown for metronidazole in mouse and rat studies, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. Alternatively, a nursing mother may choose to pump and discard human milk for the duration of metronidazole therapy, and for 24 hours after therapy ends and feed her infant stored human milk or formula.
Geriatric Use
In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
In geriatric patients, monitoring for metronidazole associated adverse events is recommended (see CLINICAL PHARMACOLOGY, PRECAUTIONS). Decreased liver function in geriatric patients can result in increased concentrations of metronidazole that may necessitate adjustment of metronidazole dosage (see DOSAGE AND ADMINISTRATION).
Pediatric Use
Pediatric Patients Less than 4 Months of Age
The safety and effectiveness of Metronidazole Injection have been established for the treatment of intraabdominal infections in pediatric patients from birth to less than 4 months. Use of Metronidazole Injection in this age group is supported by evidence from data in adults with additional pharmacokinetic and safety data in pediatric patients from birth to less than 4 months of age.
A partially randomized, open-label, phase 2/3 study of intravenous Metronidazole Injection in combination with other intravenous antibacterial drugs was conducted in 62 preterm infants (≤ 33 weeks gestational age at birth, and postnatal age <121 days) and 55 late pre-term and term infants (≥ 34 weeks gestational age at birth, and postnatal age <121 days), with complicated intra-abdominal infections. The primary objective of this study was to evaluate the safety of metronidazole-containing regimens, which were administered for up to 10 days. The adverse reactions reported in patients receiving metronidazole-containing regimens were comparable to patients receiving other antibacterial regimens in the study and generally similar to adverse reactions described in adults.
The safety and effectiveness of Metronidazole Injection in pediatric patients less than 4 months of age have not been established for (1) the treatment of anaerobic infections other than intra-abdominal infections or for (2) prophylaxis for postoperative infections (see INDICATIONS AND USAGE, CLINICAL PHARMACOLOGY, Pediatric Patients and DOSAGE AND ADMINISTRATION).
Pediatric Patients 4 Months of Age and Older
The safety and effectiveness of Metronidazole Injection in pediatric patients 4 months of age and older have not been established.
Close