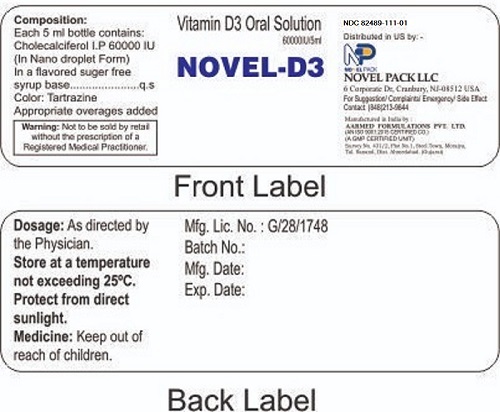
Label: NOVEL-D3- vitamin d3 solution
- NHRIC Code(s): 82489-111-01
- Packager: Novel Pack LLC
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated April 30, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- STATEMENT OF IDENTITY
- Dosage
- SAFE HANDLING WARNING
- WARNINGS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NOVEL-D3
vitamin d3 solutionProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:82489-111 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 60000 [iU] Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:82489-111-01 4 in 1 CARTON Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 04/30/2022 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color flavor Labeler - Novel Pack LLC (011480879) Establishment Name Address ID/FEI Business Operations Novel Pack LLC 011480879 label