Label: HEPARIN SODIUM injection
- NDC Code(s): 71288-400-02, 71288-400-03
- Packager: Meitheal Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 18, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use HEPARIN SODIUM INJECTION safely and effectively. See full prescribing information for HEPARIN SODIUM INJECTION. HEPARIN SODIUM ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
Heparin Sodium Injection is indicated for: Prophylaxis and treatment of venous thrombosis and pulmonary embolism; Prevention of postoperative deep venous thrombosis and pulmonary embolism in ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Preparation for Administration - Confirm the choice of the correct heparin sodium injection vial to ensure that the 1 mL vial is not confused with a “catheter lock flush” vial or other 1 mL ...
-
3 DOSAGE FORMS AND STRENGTHS
Heparin Sodium Injection, USP, preservative free, is available as follows: 2,000 USP units per 2 mL, single-dose vial
-
4 CONTRAINDICATIONS
The use of heparin sodium injection is contraindicated in patients with the following conditions: History of heparin-induced thrombocytopenia and heparin-induced thrombocytopenia and thrombosis ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Fatal Medication Errors - Do not use heparin sodium injection as a “catheter lock flush” product. Heparin sodium injection is supplied in vials containing various strengths of heparin ...
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling: Hemorrhage [see Warnings and Precautions (5.2)] Heparin-Induced Thrombocytopenia and ...
-
7 DRUG INTERACTIONS
7.1 Oral Anticoagulants - Heparin sodium may prolong the one-stage prothrombin time. Therefore, when heparin sodium is given with dicumarol or warfarin sodium, a period of at least 5 hours after ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - There are no available data on heparin sodium use in pregnant women to inform a drug-associated risk of major birth defects and miscarriage. In published ...
-
10 OVERDOSAGE
Bleeding is the chief sign of heparin overdosage. Neutralization of Heparin Effect - When clinical circumstances (bleeding) require reversal of the heparin effect, protamine sulfate (1 ...
-
11 DESCRIPTION
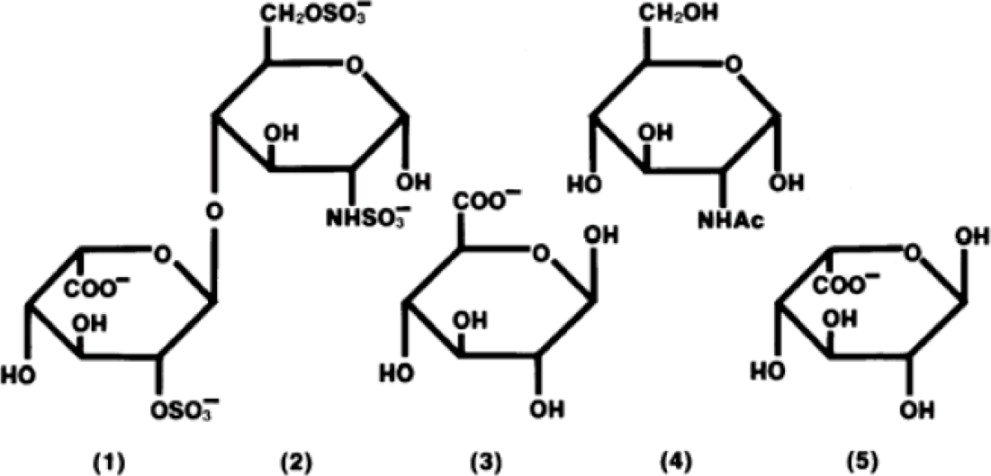
Heparin is a heterogeneous group of straight-chain anionic mucopolysaccharides, called glycosaminoglycans, having anticoagulant properties. Although others may be present, the main sugars ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Heparin interacts with the naturally occurring plasma protein, Antithrombin III, to induce a conformational change, which markedly enhances the serine protease ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No long-term studies in animals have been performed to evaluate carcinogenic potential of heparin. Also, no reproduction studies in ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Heparin Sodium Injection, USP (porcine), preservative free, is available as follows: NDCHeparin Sodium Injection, USP (1,000 USP units per mL)Package Factor - 71288-400-03 - 2,000 USP units ...
-
17 PATIENT COUNSELING INFORMATION
Hemorrhage - Inform patients that it may take them longer than usual to stop bleeding, that they may bruise and/or bleed more easily when they are treated with heparin, and that they should ...
-
Principal Display Panel – Heparin Sodium Injection, USP 2000 USP Units Vial Label
NDC 71288-400-02 - Rx Only - Heparin Sodium Injection, USP - 2,000 USP units per 2 mL - (1,000 USP units per mL) NOT for Lock Flush - Preservative-Free - For Intravenous or Subcutaneous Use
-
Principal Display Panel – Heparin Sodium Injection, USP 2000 USP Units Carton
NDC 71288-400-03 - 25 x 2 mL Single-Dose Vials - Discard unused portion - Rx Only - Heparin Sodium Injection, USP - 2,000 USP units per 2 mL - (1,000 USP units per mL) NOT for Lock ...
-
INGREDIENTS AND APPEARANCEProduct Information